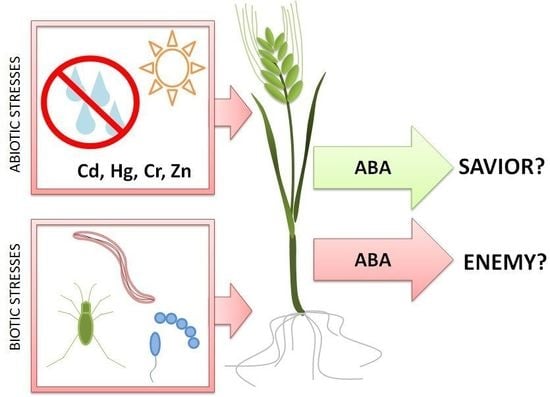
Abscisic Acid—Enemy or Savior in the Response of Cereals to Abiotic and Biotic Stresses?
Abstract
:1. Introduction
2. Abscisic Acid
2.1. ABA Metabolism
2.2. ABA Signaling in Response to Stress
2.3. Transport and Production of ABA Under Stress
3. Role of ABA in Abiotic Stresses
3.1. Resistance of Wheat and Barley to Abiotic Stress
3.2. Hormonal Plant Response to Abiotic Environmental Factors
3.3. The Role of ABA in the Physiological Response of Plants to Abiotic Stress
3.4. Impact of ABA on Seed Dormancy and Germination Under Stress
3.5. A Common ABA-Dependent Response to Abiotic Stress
3.6. Role of ABA in Protein Expression under Different Stresses
3.7. ABA Regulation in Senescence
3.8. Deleterious Consequences of ABA Accumulation
4. ABA in Cereals Responses to Biotic Stresses
4.1. Susceptibility of Wheat and Barley to Biotic Stresses
4.2. ABA in Fungual Diseases
4.3. ABA in Other Diseases
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fidler, J.; Grabowska, A.; Prabucka, B.; Więsyk, A.; Góra-Sochacka, A.; Bielawski, W.; Pojmaj, M.; Zdunek-Zastocka, E. The varied ability of grains to synthesize and catabolize ABA is one of the factors affecting dormancy and its release by after-ripening in imbibed triticale grains of cultivars with different pre-harvest sprouting susceptibilities. J. Plant Physiol. 2018, 226, 48–55. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 08, 106. [Google Scholar] [CrossRef] [Green Version]
- Gordon, C.S.; Rajagopalan, N.; Risseeuw, E.P.; Surpin, M.; Ball, F.J.; Barber, C.J.; Buhrow, L.M.; Clark, S.M.; Page, J.E.; Todd, C.D.; et al. Characterization of Triticum aestivum abscisic acid receptors and a possible role for these in mediating Fusarium head blight susceptibility in wheat. PLoS ONE 2016, 11, e0164996. [Google Scholar] [CrossRef] [Green Version]
- Kitahata, N.; Asami, T. Chemical biology of abscisic acid. J. Plant Res. 2011, 124, 549–557. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.; Bressan, R.A.; Song, C.; Zhu, J.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [Green Version]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, G.K.; Yamazaki, M.; Kobayashi, M.; Hirochika, R.; Miyao, A.; Hirochika, H. Screening of the rice viviparous mutants generated by endogenous retrotransposon tos17 insertion. Tagging of a zeaxanthin epoxidase gene and a novel ostatc gene. Plant Physiol. 2001, 125, 1248–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- North, H.M.; Almeida, A.D.; Boutin, J.-P.; Frey, A.; To, A.; Botran, L.; Sotta, B.; Marion-Poll, A. The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers: Cloning of a neoxanthin synthesis gene. Plant J. 2007, 50, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.H. Specific oxidative cleavage of carotenoids by VP14 of maize. Science 1997, 276, 1872–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Guzmán, M.; Apostolova, N.; Bellés, J.M.; Barrero, J.M.; Piqueras, P.; Ponce, M.R.; Micol, J.L.; Serrano, R.; Rodríguez, P.L. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 2002, 14, 1833–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdunek-Zastocka, E. Molecular cloning, characterization and expression analysis of three aldehyde oxidase genes from Pisum sativum L. Plant Physiol. Biochem. 2008, 46, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Kushiro, T.; Jikumaru, Y.; Abrams, S.R.; Kamiya, Y.; Seki, M.; Nambara, E. ABA 9′-hydroxylation is catalyzed by CYP707A in Arabidopsis. Phytochemistry 2011, 72, 717–722. [Google Scholar] [CrossRef] [Green Version]
- Kushiro, T.; Okamoto, M.; Nakabayashi, K.; Yamagishi, K.; Kitamura, S.; Asami, T.; Hirai, N.; Koshiba, T.; Kamiya, Y.; Nambara, E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: Key enzymes in ABA catabolism. EMBO J. 2004, 23, 1647–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Hirai, N.; Matsumoto, C.; Ohigashi, H.; Ohta, D.; Sakata, K.; Mizutani, M. Arabidopsis CYP707A s encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol. 2004, 134, 1439–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, J.-K.; Ye, M.; Li, B.; Noel, J.P. Co-evolution of hormone metabolism and signaling networks expands plant adaptive plasticity. Cell 2016, 166, 881–893. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Cutler, A.J.; Ambrose, S.J.; Galka, M.M.; Nelson, K.M.; Squires, T.M.; Loewen, M.K.; Jadhav, A.S.; Ross, A.R.S.; Taylor, D.C.; et al. A new abscisic acid catabolic pathway. Plant Physiol. 2004, 134, 361–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadhav, A.S.; Taylor, D.C.; Giblin, M.; Ferrie, A.M.R.; Ambrose, S.J.; Ross, A.R.S.; Nelson, K.M.; Irina Zaharia, L.; Sharma, N.; Anderson, M.; et al. Hormonal regulation of oil accumulation in Brassica seeds: Metabolism and biological activity of ABA, 7′-, 8′- and 9′-hydroxy ABA in microspore derived embryos of B. napus. Phytochemistry 2008, 69, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Bajguz, A. Conjugates of abscisic acid, brassinosteroids, ethylene, gibberellins, and jasmonates. Phytochemistry 2011, 72, 2097–2112. [Google Scholar] [CrossRef] [PubMed]
- Burla, B.; Pfrunder, S.; Nagy, R.; Francisco, R.M.; Lee, Y.; Martinoia, E. Vacuolar transport of abscisic acid glucosyl ester is mediated by ATP-binding cassette and proton-antiport mechanisms in Arabidopsis. Plant Physiol. 2013, 163, 1446–1458. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.-J.; Nakajima, M.; Suzuki, Y.; Yamaguchi, I. Cloning and characterization of the abscisic acid-specific glucosyltransferase gene from adzuki bean seedlings. Plant Physiol. 2002, 129, 1285–1295. [Google Scholar] [CrossRef] [Green Version]
- Lim, E.-K.; Doucet, C.J.; Hou, B.; Jackson, R.G.; Abrams, S.R.; Bowles, D.J. Resolution of (+)-abscisic acid using an Arabidopsis glycosyltransferase. Tetrahedron Asymmetry 2005, 16, 143–147. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, J.-P.; Li, D.-K.; Luo, Q.; Yan, Q.; Liu, Z.-B.; Ye, L.-M.; Wang, J.-M.; Li, X.-F.; Yang, Y. UDP-glucosyltransferase 71c5, a major glucosyltransferase, mediates abscisic acid homeostasis in Arabidopsis. Plant Physiol. 2015, 167, 1659–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, F.; Hartung, W. Long-distance signalling of abscisic acid (ABA): The factors regulating the intensity of the ABA signal. J. Exp. Bot. 2007, 59, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.H.; Piao, H.L.; Kim, H.-Y.; Choi, S.M.; Jiang, F.; Hartung, W.; Hwang, I.; Kwak, J.M.; Lee, I.-J.; Hwang, I. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 2006, 126, 1109–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.-Y.; Kim, D.H.; Hwang, I. ABA homeostasis and signaling involving multiple subcellular compartments and multiple receptors. Plant Cell Rep. 2013, 32, 807–813. [Google Scholar] [CrossRef]
- Abscisic Acid: Metabolism, Transport and Signaling; Zhang, D.-P. (Ed.) Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-017-9423-7. [Google Scholar]
- Seiler, C.; Harshavardhan, V.T.; Reddy, P.S.; Hensel, G.; Kumlehn, J.; Eschen-Lippold, L.; Rajesh, K.; Korzun, V.; Wobus, U.; Lee, J.; et al. Abscisic acid flux alterations result in differential abscisic acid signaling responses and impact assimilation efficiency in barley under terminal drought stress. Plant Physiol. 2014, 164, 1677–1696. [Google Scholar] [CrossRef]
- Hwang, S.-G.; Chen, H.-C.; Huang, W.-Y.; Chu, Y.-C.; Shii, C.-T.; Cheng, W.-H. Ectopic expression of rice OsNCED3 in Arabidopsis increases ABA level and alters leaf morphology. Plant Sci. 2010, 178, 12–22. [Google Scholar] [CrossRef]
- Ji, X.; Dong, B.; Shiran, B.; Talbot, M.J.; Edlington, J.E.; Hughes, T.; White, R.G.; Gubler, F.; Dolferus, R. Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiol. 2011, 156, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Mega, R.; Meguro-Maoka, A.; Endo, A.; Shimosaka, E.; Murayama, S.; Nambara, E.; Seo, M.; Kanno, Y.; Abrams, S.R.; Sato, Y. Sustained low abscisic acid levels increase seedling vigor under cold stress in rice (Oryza sativa L.). Sci. Rep. 2015, 5, 13819. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 2009, 1172408. [Google Scholar] [CrossRef]
- Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.-F.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 1173041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyakawa, T.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Tanokura, M. Structure and function of abscisic acid receptors. Trends Plant Sci. 2013, 18, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Jiang, L.; Xin, Q.; Liu, Y.; Tan, J.X.; Chen, Z.Z. Structural basis and functions of abscisic acid receptors PYLs. Front. Plant Sci. 2015, 6, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Kong, L.; Zhou, Y.; Chen, Z.; Tian, D.; Lin, Y.; Wang, F.; Chen, S. Endosperm-specific OsPYL8 and OsPYL9 act as positive regulators of the ABA signaling pathway in rice seed germination. Functional Plant Biol. 2017, 44, 635. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, Z.; Li, X.; Lv, T.; Liu, H.; Wang, L.; Niu, H.; Bu, Q. Characterization and functional analysis of pyrabactin resistance-like abscisic acid receptor family in rice. Rice 2015, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.T.C.; Lee, S.; Choi, S.; Na, Y.; Song, M.; Hoang, Q.T.N.; Sim, S.Y.; Kim, M.-S.; Kim, J.-I.; Soh, M.-S.; et al. Arabidopsis raf-like kinase Raf10 is a regulatory component of core ABA signaling. Mol. Cells 2019, 42, 646. [Google Scholar] [CrossRef]
- Umezawa, T.; Sugiyama, N.; Mizoguchi, M.; Hayashi, S.; Myouga, F.; Yamaguchi-Shinozaki, K.; Ishihama, Y.; Hirayama, T.; Shinozaki, K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 17588. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic atress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Li, J. Proteomic analysis of phosphoproteins regulated by abscisic acid in rice leaves. Biochem. Biophys. Res. Commun. 2008, 371, 883–888. [Google Scholar] [CrossRef]
- Mega, R.; Abe, F.; Kim, J.-S.; Tsuboi, Y.; Tanaka, K.; Kobayashi, H.; Sakata, Y.; Hanada, K.; Tsujimoto, H.; Kikuchi, J.; et al. Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nature Plants 2019, 5, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, X.; Song, J.; Zong, X.; Li, D.; Li, D. Over-expression of a Zea mays L. protein phosphatase 2C gene (ZmPP2C) in Arabidopsis thaliana decreases tolerance to salt and drought. J. Plant Physiol. 2009, 166, 531–542. [Google Scholar] [CrossRef]
- Hu, X.; Liu, L.; Xiao, B.; Li, D.; Xing, X.; Kong, X.; Li, D. Enhanced tolerance to low temperature in tobacco by over-expression of a new maize protein phosphatase 2C, ZmPP2C2. J. Plant Physiol. 2010, 167, 1307–1315. [Google Scholar] [CrossRef]
- Singh, A.; Jha, S.K.; Bagri, J.; Pandey, G.K. ABA inducible rice protein phosphatase 2C confers ABA insensitivity and abiotic stress tolerance in Arabidopsis. PLoS ONE 2015, 10, e0125168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Pandey, A.; Srivastava, A.K.; Tran, L.-S.P.; Pandey, G.K. Plant protein phosphatases 2C: From genomic diversity to functional multiplicity and importance in stress management. Crit. Rev. Biotechnol. 2016, 36, 1023–1035. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Mao, X.; Jing, R.; Jia, H. Differential activation of the wheat SnRK2 family by abiotic stresses. Front. Plant Sci. 2016, 7, 420. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Zhang, H.; Tian, S.; Chang, X.; Jing, R. TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J. Exp. Bot. 2010, 61, 683–696. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Mao, X.; Wang, C.; Jing, R. Overexpression of a common wheat gene TaSnRK2.8 enhances tolerance to drought, salt and low temperature in Arabidopsis. PLoS ONE 2010, 5, e16041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christmann, A.; Hoffmann, T.; Teplova, I.; Grill, E.; Müller, A. Generation of active pools of abscisic acid revealed by in vivo imaging of water-stressed Arabidopsis. Plant Physiol. 2005, 137, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, K.J.; Sauter, A.; Wichert, K.; Messdaghi, D.; Hartung, W. Extracellular beta-glucosidase activity in barley involved in the hydrolysis of ABA glucose conjugate in leaves. J. Exp. Bot. 2000, 51, 937–944. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Planchon, S.; Renaut, J.; Vanková, R.; Prášil, I.T. Proteome analysis of cold response in spring and winter wheat (Triticum aestivum) crowns reveals similarities in stress adaptation and differences in regulatory processes between the growth habits. J. Proteome Res. 2013, 12, 4830–4845. [Google Scholar] [CrossRef]
- Sembdner, G.; Atzorn, R.; Schneider, G. Plant hormone conjugation. Plant Mol. Biol. 1994, 26, 1459–1481. [Google Scholar] [CrossRef]
- Thameur, A.; Ferchichi, A.; López-Carbonell, M. Quantification of free and conjugated abscisic acid in five genotypes of barley (Hordeum vulgare L.) under water stress conditions. S. Afr. J. Bot. 2011, 77, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Kudoyarova, G.; Veselova, S.; Hartung, W.; Farhutdinov, R.; Veselov, D.; Sharipova, G. Involvement of root ABA and hydraulic conductivity in the control of water relations in wheat plants exposed to increased evaporative demand. Planta 2011, 233, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Vanhaelewyn, L.; Prinsen, E.; Van Der Straeten, D.; Vandenbussche, F. Hormone-controlled UV-B responses in plants. J. Exp. Bot. 2016, 67, 4469–4482. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data (accessed on 28 June 2020).
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef] [Green Version]
- Zagdańska, B. Mechanizmy odpornosci zboz na susze glebowa: Metabolizm energetyczny pszenicy jarej w nabywaniu odpornosci. Biuletyn Instytutu Hodowli i Aklimatyzacji Roślin 1997, 203, 41–55. [Google Scholar]
- Boussora, F.; Allam, M.; Guasmi, F.; Ferchichi, A.; Rutten, T.; Hansson, M.; Youssef, H.M.; Börner, A. Spike developmental stages and ABA role in spikelet primordia abortion contribute to the final yield in barley (Hordeum vulgare L.). Bot. Stud. 2019, 60, 13. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Díaz, M.; García, J.L.; Antolín, M.C.; Araus, J.L. Effects of soil drought and atmospheric humidity on yield, gas exchange, and stable carbon isotope composition of barley. Photosynthetica 2002, 40, 415–421. [Google Scholar] [CrossRef]
- Balla, K.; Karsai, I.; Bónis, P.; Kiss, T.; Berki, Z.; Horváth, Á.; Mayer, M.; Bencze, S.; Veisz, O. Heat stress responses in a large set of winter wheat cultivars (Triticum aestivum L.) depend on the timing and duration of stress. PLoS ONE 2019, 14, e0222639. [Google Scholar] [CrossRef]
- Mahmood, T.; Islam, R.; Muhammad, A. Toxic effects of heavy metals on early growth and tolerance of cereal crops. Pak. J. Bot. 2007, 39, 451–462. [Google Scholar]
- Gang, A.; Vyas, A.; Vyas, H.K. Toxic effect of heavy metals on germination and seedling growth of wheat. J. Environ. Res. Dev. 2013, 8, 206–213. [Google Scholar]
- Ahmad, A.; Aref, I.M.; Owen, G. Tolerance capacity of Turkish genotypes of barley (Hordeum vulagare L.) for cadmium stress. J. Environ. Biol. 2018, 39, 1027–1035. [Google Scholar] [CrossRef]
- Kacperska, A. Udział hormonów roślinnych w odpowiedzi roślin na stresowe czynniki środowiska. Kosmos 1995, 44, 623–637. [Google Scholar]
- Kudoyarova, G.R.; Veselov, D.S.; Sharipova, G.V.; Akhiyarova, G.R.; Dodd, I.C.; Veselov, S.Y. Water relations and growth of original barley plants and its ABA-deficient mutants at increased air temperature. Russ. J. Plant Physiol. 2014, 61, 188–193. [Google Scholar] [CrossRef]
- Moussa, H.R.; El-Gamal, S.M. Effect of salicylic acid pretreatment on cadmium toxicity in wheat. Biol. Plant. 2010, 54, 315–320. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Zu, Y. Intraspecific variation in sensitivity to ultraviolet-B radiation in endogenous hormones and photosynthetic characteristics of 10 wheat cultivars grown under field conditions. S. Afr. J. Bot. 2010, 76, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Ma, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of exogenous Ca2+ on phenolic accumulation and physiological changes in germinated wheat (Triticum aestivum L.) under UV-B radiation. Food Chem. 2019, 288, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Klink, K.; Wiersma, J.J.; Crawford, C.J.; Stuthman, D.D. Impacts of temperature and precipitation variability in the Northern Plains of the United States and Canada on the productivity of spring barley and oat. Int. J. Climatol. 2014, 34, 2805–2818. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Basu, S.; Kumar, S.; Kumar, G.; Prakash, V.; Kumar, S.; Mishra, J.S.; Bhatt, B.P.; Malviya, N.; Singh, G.P.; et al. Heat stress induced impairment of starch mobilisation regulates pollen viability and grain yield in wheat: Study in Eastern Indo-Gangetic Plains. Field Crops Res. 2017, 206, 106–114. [Google Scholar] [CrossRef]
- Ayers, A.D.; Brown, J.W.; Wadleigh, C.H. Salt tolerance of barley and wheat in soil plots receiving several salinization regimes. Agron. J. 1952, 44, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Maas, E.V.; Hoffman, G.J.; Chaba, G.D.; Poss, J.A.; Shannon, M.C. Salt sensitivity of corn at various growth stages. Irrig. Sci. 1983, 4, 45–57. [Google Scholar] [CrossRef]
- Maas, E.V.; Poss, J.A. Salt sensitivity of wheat at various growth stages. Irrig. Sci. 1989, 10, 29–40. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poorter, H. Plant growth analysis: Towards a synthesis of the classical and the functional approach. Physiol. Plant. 1989, 75, 237–244. [Google Scholar] [CrossRef]
- Agricultural Salinity Assessment and Management, 2nd ed.; Wallender, W.W.; Tanji, K.K. (Eds.) American Society of Civil Engineers: Reston, VA, USA, 2011; ISBN 978-0-7844-1169-8. [Google Scholar]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Rebekić, A.; Lončarić, Z. Genotypic difference in cadmium effect on agronomic traits and grain zinc and iron concentration in winter wheat. Emir. J. Food Agric. 2016, 28, 772–778. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 08, 475. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.; Sebastian, J.; Dinneny, J.R. Salt-stress regulation of root system growth and architecture in Arabidopsis seedlings. In Plant Cell Expansion; Estevez, J.M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1242, pp. 105–122. ISBN 978-1-4939-1901-7. [Google Scholar]
- De Smet, I.; Signora, L.; Beeckman, T.; Inzé, D.; Foyer, C.H.; Zhang, H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 2003, 33, 543–555. [Google Scholar] [CrossRef] [Green Version]
- Deak, K.; Malamy, J. Osmotic regulation of root system architecture. Plant J. 2005, 43, 17–28. [Google Scholar] [CrossRef]
- Ktitorova, I.N.; Skobeleva, O.V.; Kanash, E.V.; Bilova, T.E.; Sharova, E.I. Causes of root growth retardation induced by ultraviolet-B irradiation of shoots in barley seedlings. Russ. J. Plant Physiol. 2006, 53, 85–95. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, R.-G.; Mao, G.; Koczan, J.M. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol. 2006, 142, 1065–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyers, J.D.B.; Paterson, N.W. Plant hormones and the control of physiological processes. New Phytol. 2001, 152, 375–407. [Google Scholar] [CrossRef] [Green Version]
- Graeber, K.; Linkies, A.; Müller, K.; Wunchova, A.; Rott, A.; Leubner-Metzger, G. Cross-species approaches to seed dormancy and germination: Conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes. Plant Mol. Biol. 2010, 73, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Suzuki, M.; Ketterling, M.G.; Li, Q.-B.; McCarty, D.R. Viviparous1 alters global gene expression patterns through regulation of abscisic acid signaling. Plant Physiol. 2003, 132, 1664–1677. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Costa, L.M.; Biderre-Petit, C.; Kbhaya, B.; Dey, N.; Perez, P.; McCarty, D.R.; Gutierrez-Marcos, J.F.; Becraft, P.W. Abscisic acid and stress signals induce Viviparous1 expression in seed and vegetative tissues of maize. Plant Physiol. 2007, 143, 720–731. [Google Scholar] [CrossRef]
- McKibbin, R.S.; Wilkinson, M.D.; Bailey, P.C.; Flintham, J.E.; Andrew, L.M.; Lazzeri, P.A.; Gale, M.D.; Lenton, J.R.; Holdsworth, M.J. Transcripts of Vp-1 homeologues are misspliced in modern wheat and ancestral species. Proc. Nat. Acad. Sci. USA 2002, 99, 10203–10208. [Google Scholar] [CrossRef] [Green Version]
- Hauvermale, A.L.; Ariizumi, T.; Steber, C.M. Gibberellin signaling: A theme and variations on DELLA repression. Plant Physiol. 2012, 160, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Leymarie, J.; Benech-Arnold, R.L.; Farrant, J.M.; Corbineau, F. Thermodormancy and ABA metabolism in barley grains. Plant Signal. Behav. 2009, 4, 205–207. [Google Scholar] [CrossRef] [Green Version]
- Fidler, J.; Zdunek-Zastocka, E.; Bielawski, W. Regulation of abscisic acid metabolism in relation to the dormancy and germination of cereal grains. Acta Soc. Bot. Pol. 2015, 84, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasulu, N.; Borisjuk, L.; Junker, B.; Mock, H.-P.; Rolletschek, H.; Seiffert, U.; Weschke, W.; Wobus, U. Barley grain development: Toward an integrative view. Int. Rev. Cell Mol. Biol. 2010, 281, 49–89. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, L.M.C.; Oplaat, C.; Feron, R.; Ma, Q.; Wolters-Arts, M.; Hedden, P.; Mariani, C.; Vriezen, W.H. Abscisic acid levels in tomato ovaries are regulated by LeNCED1 and SlCYP707A1. Planta 2009, 229, 1335–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sairam, R.K.; Tyagi, A. Physiology and molecular biology of salinity stress tolerance in plants. Curr. Sci. 2004, 86, 407–421. [Google Scholar]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange: Brassinolide improves drought tolerance in maize. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Bagues, M.; Sarabi, B.; Ghashghaie, J.; Souli, I.; Nagaz, K. The validity of carbon isotope discrimination as a screening criterion for grain yield in two barley landraces under deficit irrigation with saline water in southern Tunisia. Plant Biotechnol. 2018, 35, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.-H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [Green Version]
- Muszyńska, E.; Labudda, M. Dual role of metallic trace elements in stress biology—From negative to beneficial impact on plants. Int. J. Mol. Sci. 2019, 20, 3117. [Google Scholar] [CrossRef] [Green Version]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, M.A. Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193–199. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Allen, G.J.; Hugouvieux, V.; Kwak, J.M.; Waner, D. Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 627–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.-M.; Zhao, Z.; Assmann, S.M. Guard cells: A dynamic signaling model. Curr. Opin. Plant Biol. 2004, 7, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.-M.; Kuchitsu, K. Early ABA signaling events in guard cells. J. Plant Growth. Regul. 2005, 24, 296–307. [Google Scholar] [CrossRef]
- Hollenbach, B.; Schreiber, L.; Hartung, W.; Dietz, K.-J. Cadmium leads to stimulated expression of the lipid transfer protein genes in barley: Implications for the involvement of lipid transfer proteins in wax assembly. Planta 1997, 203, 9–19. [Google Scholar] [CrossRef]
- Salminen, T.A.; Blomqvist, K.; Edqvist, J. Lipid transfer proteins: Classification, nomenclature, structure, and function. Planta 2016, 244, 971–997. [Google Scholar] [CrossRef] [Green Version]
- Balota, M.; Payne, W.A.; Evett, S.R.; Peters, T.R. Morphological and physiological traits associated with canopy temperature depression in three closely related wheat lines. Crop Sci. 2008, 48, 1897–1910. [Google Scholar] [CrossRef] [Green Version]
- Shakirova, F.M.; Allagulova, C.R.; Maslennikova, D.R.; Klyuchnikova, E.O.; Avalbaev, A.M.; Bezrukova, M.V. Salicylic acid-induced protection against cadmium toxicity in wheat plants. Environ. Exp. Bot. 2016, 122, 19–28. [Google Scholar] [CrossRef]
- Zeeshan, M.; Lu, M.; Naz, S.; Sehar, S.; Cao, F.; Wu, F. Resemblance and difference of seedling metabolic and transporter gene expression in high tolerance wheat and barley cultivars in response to salinity stress. Plants 2020, 9, 519. [Google Scholar] [CrossRef]
- Du, H.; Wu, N.; Chang, Y.; Li, X.; Xiao, J.; Xiong, L. Carotenoid deficiency impairs ABA and IAA biosynthesis and differentially affects drought and cold tolerance in rice. Plant Mol. Biol. 2013, 83, 475–488. [Google Scholar] [CrossRef]
- Maurel, C.; Verdoucq, L.; Luu, D.-T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosová, K.; Prášil, I.T.; Vítámvás, P.; Dobrev, P.; Motyka, V.; Floková, K.; Novák, O.; Turečková, V.; Rolčik, J.; Pešek, B.; et al. Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J. Plant Physiol. 2012, 169, 567–576. [Google Scholar] [CrossRef]
- Hu, X.-J.; Chen, D.; Lynne Mclntyre, C.; Fernanda Dreccer, M.; Zhang, Z.-B.; Drenth, J.; Kalaipandian, S.; Chang, H.; Xue, G.-P. Heat shock factor C2a serves as a proactive mechanism for heat protection in developing grains in wheat via an ABA-mediated regulatory pathway: TaHsfC2a plays a proactive heat protection role. Plant Cell Environ. 2018, 41, 79–98. [Google Scholar] [CrossRef]
- Ergün, N.; Öncel, I. Effects of some heavy metals and heavy metal hormone interactions on wheat (Triticum aestivum L. cv. Gun 91) seedlings. Afr. J. Agric. Res. 2012, 7, 1518–1523. [Google Scholar] [CrossRef]
- Ci, D.; Jiang, D.; Dai, T.; Jing, Q.; Cao, W. Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere 2009, 77, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhang, B.B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E671–E678. [Google Scholar] [CrossRef] [Green Version]
- Guan, L.M.; Zhao, J.; Scandalios, J.G. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 2000, 22, 87–95. [Google Scholar] [CrossRef]
- Agarwal, S.; Sairam, R.K.; Srivastava, G.C.; Tyagi, A.; Meena, R.C. Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Sci. 2005, 169, 559–570. [Google Scholar] [CrossRef]
- Milla, M.A.R.; Maurer, A.; Huete, A.R.; Gustafson, J.P. Glutathione peroxidase genes in Arabidopsis are ubiquitous and regulated by abiotic stresses through diverse signaling pathways: Glutathione peroxidase genes in Arabidopsis. Plant J. 2003, 36, 602–615. [Google Scholar] [CrossRef]
- Moons, A. Osgstu3 and osgtu4, encoding tau class glutathione S-transferases, are heavy metal- and hypoxic stress-induced and differentially salt stress-responsive in rice roots. FEBS Lett. 2003, 553, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Szypulska, E.; Jankowski, K.; Weidner, S. ABA pretreatment can limit salinity-induced proteome changes in growing barley sprouts. Acta Physiol. Plant. 2017, 39, 190. [Google Scholar] [CrossRef] [Green Version]
- Szypulska, E.; Weidner, S. Importance of cytomatrix-bound polysomes to synthesis of lysine-containing proteins in triticale germs under ABA treatment. Acta Physiol. Plant. 2011, 33, 1461–1465. [Google Scholar] [CrossRef] [Green Version]
- Xue, G.-P.; Loveridge, C.W. HvDRF1 is involved in abscisic acid-mediated gene regulation in barley and produces two forms of AP2 transcriptional activators, interacting preferably with a CT-rich element. Plant J. 2004, 37, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Schoonheim, P.J.; Sinnige, M.P.; Casaretto, J.A.; Veiga, H.; Bunney, T.D.; Quatrano, R.S.; de Boer, A.H. 14-3-3 adaptor proteins are intermediates in ABA signal transduction during barley seed germination. Plant J. 2007, 49, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-J.; Ho, T.-H. An abscisic acid-induced protein, HVA22, inhibits gibberellin-mediated programmed cell death in cereal aleurone cells. Plant Physiol. 2008, 147, 1710–1722. [Google Scholar] [CrossRef] [Green Version]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The role and regulation of ABI5 (ABA-Insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef] [Green Version]
- Tamás, L.; Mistrík, I.; Huttová, J.; Halušková, L.; Valentovičová, K.; Zelinová, V. Role of reactive oxygen species-generating enzymes and hydrogen peroxide during cadmium, mercury and osmotic stresses in barley root tip. Planta 2009, 231, 221. [Google Scholar] [CrossRef]
- Maslennikova, D.; Fatkhutdinova, R.A.; Bezrukova, M.; Chulpan, A.; Klyuchnikova, E.O.; Shakirova, F.M. Mechanisms of protective action of salicylic acid on wheat plants under cadmium stress. Agrokhimiya 2013, 3, 72–79. [Google Scholar]
- Allagulova, C.R.; Maslennikova, D.R.; Avalbaev, A.M.; Fedorova, K.A.; Yuldashev, R.A.; Shakirova, F.M. Influence of 24-epibrassinolide on growth of wheat plants and the content of dehydrins under cadmium stress. Russ. J. Plant Physiol. 2015, 62, 465–471. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T. Wheat and barley dehydrins under cold, drought, and salinity—what can LEA-II proteins tell us about plant stress response? Front. Plant Sci. 2014, 5, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, D.; Shekhar, S.; Singh, D.; Chakraborty, S.; Chakraborty, N. Heat shock proteins and abiotic stress tolerance in plants. In Regulation of Heat Shock Protein Responses; Asea, A.A.A., Kaur, P., Eds.; Heat Shock Proteins; Springer International Publishing: Cham, Switzerland, 2018; Volume 13, pp. 41–69. ISBN 978-3-319-74714-9. [Google Scholar]
- Xue, G.-P.; Sadat, S.; Drenth, J.; McIntyre, C.L. The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J. Exp. Bot. 2014, 65, 539–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker-Simmons, M.; Sesing, J. Temperature effects on embryonic abscisic acid levels during development of wheat grain dormancy. J. Plant Growth Regul. 1990, 9, 51–56. [Google Scholar] [CrossRef]
- Fragkostefanakis, S.; Röth, S.; Schleiff, E.; Scharf, K.-D. Prospects of engineering thermotolerance in crops through modulation of heat stress transcription factor and heat shock protein networks: Hsfs and Hsps for improvement of crop thermotolerance. Plant Cell Environ. 2015, 38, 1881–1895. [Google Scholar] [CrossRef]
- Panikulangara, T.J.; Eggers-Schumacher, G.; Wunderlich, M.; Stransky, H.; Schöffl, F. Galactinol synthase 1. A novel heat shock factor target gene responsible for heat-induced synthesis of raffinose family oligosaccharides in Arabidopsis. Plant Physiol. 2004, 136, 3148–3158. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, F.; Guy, C.L. β-Amylase induction and the protective role of maltose during temperature shock. Plant Physiol. 2004, 135, 1674–1684. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Jiang, H.; Zhao, Z.; An, L. Abscisic acid is involved in brassinosteroids-induced chilling tolerance in the suspension cultured cells from Chorispora bungeana. J. Plant Physiol. 2011, 168, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, L.; Yang, Y.; Wang, P.; Guo, T.; Kang, G. Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front. Plant Sci. 2015, 6, 458. [Google Scholar] [CrossRef]
- Ferrandino, A.; Lovisolo, C. Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ. Exp. Bot. 2014, 103, 138–147. [Google Scholar] [CrossRef]
- Rakić, T.; Lazarević, M.; Jovanović, Z.S.; Radović, S.; Siljak-Yakovlev, S.; Stevanović, B.; Stevanović, V. Resurrection plants of the genus Ramonda: Prospective survival strategies—Unlock further capacity of adaptation, or embark on the path of evolution? Front. Plant Sci. 2014, 4, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidyanathan, H.; Sivakumar, P.; Chakrabarty, R.; Thomas, G. Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.)—differential response in salt-tolerant and sensitive varieties. Plant Sci. 2003, 165, 1411–1418. [Google Scholar] [CrossRef]
- Eltayeb, A.E.; Kawano, N.; Badawi, G.H.; Kaminaka, H.; Sanekata, T.; Morishima, I.; Shibahara, T.; Inanaga, S.; Tanaka, K. Enhanced tolerance to ozone and drought stresses in transgenic tobacco overexpressing dehydroascorbate reductase in cytosol. Physiol. Plant. 2006, 127, 57–65. [Google Scholar] [CrossRef]
- Gietler, M.; Nykiel, M.; Zagdańska, B.M. Changes in the reduction state of ascorbate and glutathione, protein oxidation and hydrolysis leading to the development of dehydration intolerance in Triticum aestivum L. seedlings. Plant Growth Regul. 2016, 79, 287–297. [Google Scholar] [CrossRef] [Green Version]
- Tossi, V.; Lamattina, L.; Cassia, R. An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol. 2009, 181, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Berli, F.J.; Moreno, D.; Piccoli, P.; Hespanhol-Viana, L.; Silva, M.F.; Bressan-Smith, R.; Cavagnaro, J.B.; Bottini, R. Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ. 2010, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jukanti, A.K.; Fischer, A.M. A high-grain protein content locus on barley (Hordeum vulgare) chromosome 6 is associated with increased flag leaf proteolysis and nitrogen remobilization. Physiol. Plant. 2008, 132, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Gan, S.-S. An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves. Plant Physiol. 2012, 158, 961–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roulin, S.; Feller, U. Light-independent degradation of stromal proteins in intact chloroplasts isolated from Pisum sativum L. leaves: Requirement for divalent cations. Planta 1998, 205, 297–304. [Google Scholar] [CrossRef]
- Akter, N.; Rafiqul Islam, M. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37, 37. [Google Scholar] [CrossRef]
- Valizadeh-Kamran, R.; Toorchi, M.; Mogadam, M.; Mohammadi, H.; Pessarakli, M. Effects of freeze and cold stress on certain physiological and biochemical traits in sensitive and tolerant barley (Hordeum vulgare) genotypes. J. Plant Nutr. 2018, 41, 102–111. [Google Scholar] [CrossRef]
- Wang, X.; Ma, R.; Cui, D.; Cao, Q.; Shan, Z.; Jiao, Z. Physio-biochemical and molecular mechanism underlying the enhanced heavy metal tolerance in highland barley seedlings pre-treated with low-dose gamma irradiation. Sci. Rep. 2017, 7, 14233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, S.; Lata, C. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Lv, B.; Luo, L.; He, J.; Mao, C.; Xi, D.; Ming, F. The NAC-type transcription factor OsNAC2 regulates ABA-dependent genes and abiotic stress tolerance in rice. Sci. Rep. 2017, 7, 40641. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Lu, S.; Lv, B.; Zhang, B.; Shen, J.; He, J.; Luo, L.; Xi, D.; Chen, X.; Ming, F. A rice NAC transcription factor promotes leaf senescence via ABA biosynthesis. Plant Physiol. 2017, 174, 1747–1763. [Google Scholar] [CrossRef] [Green Version]
- Yamburenko, M.V.; Zubo, Y.O.; Vanková, R.; Kusnetsov, V.V.; Kulaeva, O.N.; Börner, T. Abscisic acid represses the transcription of chloroplast genes. J. Exp. Bot. 2013, 64, 4491–4502. [Google Scholar] [CrossRef] [Green Version]
- Gan, S. Mitotic and postmitotic senescence in plants. Sci. Aging Knowl. Environ. 2003, 2003, 7. [Google Scholar] [CrossRef] [Green Version]
- Passioura, J. The drought environment: Physical, biological and agricultural perspectives. J. Exp. Bot. 2007, 58, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Asthir, B.; Bhatia, S. In vivo studies on artificial induction of thermotolerance to detached panicles of wheat (Triticum aestivum L.) cultivars under heat stress. J. Food Sci. Technol 2014, 51, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Shiran, B.; Wan, J.; Lewis, D.C.; Jenkins, C.L.D.; Condon, A.G.; Richards, R.A.; Dolferus, R. Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat: Reproductive stage drought tolerance in wheat. Plant Cell Environ. 2010, 33, 926–942. [Google Scholar] [CrossRef]
- Sharma, K.D.; Nayyar, H. Regulatory networks in pollen development under cold stress. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Savary, S.; Ficke, A.; Aubertot, J.-N.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Sec. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Randhawa, M.S.; Bhavani, S.; Singh, P.K.; Huerta-Espino, J.; Singh, R.P. Disease resistance in wheat: Present status and future prospects. In Disease Resistance in Crop Plants; Wani, S.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 61–81. ISBN 978-3-030-20727-4. [Google Scholar]
- Shamanin, V.; Shepelev, S.; Pozherukova, V.; Gultyaeva, E.; Kolomiets, T.; Pakholkova, E.; Morgounov, A. Primary hexaploid synthetics: Novel sources of wheat disease resistance. Crop Prot. 2019, 121, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Mehta, S.; Aggarwal, S.K.; Tiwari, M.; Bhuyan, S.I.; Bhatia, S.; Islam, M.A. Barley, disease resistance, and molecular breeding approaches. In Disease Resistance in Crop Plants; Wani, S.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 261–299. ISBN 978-3-030-20727-4. [Google Scholar]
- Harwood, W.A. An introduction to barley: The crop and the model. In Barley; Harwood, W.A., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1900, pp. 1–5. ISBN 978-1-4939-8942-3. [Google Scholar]
- Vos, I.A.; Verhage, A.; Schuurink, R.C.; Watt, L.G.; Pieterse, C.M.J.; Van Wees, S.C.M. Onset of herbivore-induced resistance in systemic tissue primed for jasmonate-dependent defenses is activated by abscisic acid. Front. Plant Sci. 2013, 4, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mine, A.; Berens, M.L.; Nobori, T.; Anver, S.; Fukumoto, K.; Winkelmüller, T.M.; Takeda, A.; Becker, D.; Tsuda, K. Pathogen exploitation of an abscisic acid- and jasmonate-inducible MAPK phosphatase and its interception by Arabidopsis immunity. Proc. Natl. Acad. Sci. USA 2017, 114, 7456–7461. [Google Scholar] [CrossRef] [Green Version]
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Ziegler, J.; Nobori, T.; Nair, A.; Krüler, V.; Winkelmüller, T.M.; Wang, Y.; Mine, A.; et al. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364–2373. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.; Wu, W.; Wang, K.; Zhang, Y.; Hu, Z.; Brosché, M.; Liu, S.; Overmyer, K. Cell death regulation but not abscisic acid signaling is required for enhanced immunity to Botrytis in Arabidopsis cuticle-permeable mutants. J. Exp. Bot. 2019, 70, 5971–5984. [Google Scholar] [CrossRef]
- Liu, X.; Afrin, T.; Pajerowska-Mukhtar, K.M. Arabidopsis GCN2 kinase contributes to ABA homeostasis and stomatal immunity. Commun. Biol. 2019, 2, 302. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Hu, X.; Ma, J.; Hettenhausen, C.; Wang, L.; Sun, G.; Wu, J.; Wu, J. Requirement of ABA signalling-mediated stomatal closure for resistance of wild tobacco to Alternaria alternata. Plant Pathol. 2014, 63, 1070–1077. [Google Scholar] [CrossRef]
- Kusajima, M.; Okumura, Y.; Fujita, M.; Nakashita, H. Abscisic acid modulates salicylic acid biosynthesis for systemic acquired resistance in tomato. Biosci. Biotechnol. Biochem. 2017, 81, 1850–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Huang, Z.; Li, X.; Ma, L.; Gu, Q.; Wu, H.; Liu, J.; Borriss, R.; Wu, Z.; Gao, X. Stomatal closure and SA-, JA/ET-signaling pathways are essential for Bacillus amyloliquefaciens FZB42 to restrict leaf disease caused by Phytophthora nicotianae in Nicotiana benthamiana. Front. Microbiol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, I.-M.; Venkidasamy, B.; Upadhyaya, C.P.; Packiaraj, G.; Rajakumar, G.; Thiruvengadam, M. Alleviation of Phytophthora infestans mediated necrotic stress in the transgenic potato (Solanum tuberosum L.) with enhanced ascorbic acid accumulation. Plants 2019, 8, 365. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xue, X.; Yu, Y.; Xu, M.; Lu, C.; Meng, X.; Zhang, B.; Ding, X.; Chu, Z. Copper ions suppress abscisic acid biosynthesis to enhance defence against Phytophthora infestans in potato. Mol. Plant Pathol. 2020, mpp.12919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugano, S.; Sugimoto, T.; Takatsuji, H.; Jiang, C.-J. Induction of resistance to Phytophthora sojae in soyabean (Glycine max) by salicylic acid and ethylene. Plant Pathol. 2013, 62, 1048–1056. [Google Scholar] [CrossRef]
- Chapman, K.M.; Marchi-Werle, L.; Hunt, T.E.; Heng-Moss, T.M.; Louis, J. Abscisic and jasmonic acids contribute to soybean tolerance to the soybean aphid (Aphis glycines Matsumura). Sci. Rep. 2018, 8, 15148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alazem, M.; Widyasari, K.; Kim, K.-H. An avirulent strain of soybean mosaic virus reverses the defensive effect of abscisic acid in a susceptible soybean cultivar. Viruses 2019, 11, 879. [Google Scholar] [CrossRef] [Green Version]
- Hohenstein, J.D.; Studham, M.E.; Klein, A.; Kovinich, N.; Barry, K.; Lee, Y.-J.; MacIntosh, G.C. Transcriptional and chemical changes in soybean leaves in response to long-term aphid colonization. Front. Plant Sci. 2019, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Mena, E.; Stewart, S.; Montesano, M.; Ponce de León, I. Soybean stem canker caused by Diaporthe caulivora; Pathogen diversity, colonization process, and plant defense activation. Front. Plant Sci. 2020, 10, 1733. [Google Scholar] [CrossRef]
- Asselbergh, B.; De Vleesschauwer, D.; Höfte, M. Global switches and fine-tuning—ABA modulates plant pathogen defense. MPMI 2008, 21, 709–719. [Google Scholar] [CrossRef] [Green Version]
- Ulferts, S.; Delventhal, R.; Splivallo, R.; Karlovsky, P.; Schaffrath, U. Abscisic acid negatively interferes with basal defence of barley against Magnaporthe oryzae. BMC Plant Biol. 2015, 15, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiese, J.; Kranz, T.; Schubert, S. Induction of pathogen resistance in barley by abiotic stress. Plant Biol. 2004, 6, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.-F.; Balcerzak, M.; Rocheleau, H.; Leung, W.; Wei, Y.-M.; Zheng, Y.-L.; Ouellet, T. Jasmonic acid and abscisic acid play important roles in host–pathogen interaction between Fusarium graminearum and wheat during the early stages of fusarium head blight. Physiol. Mol. Plant Path. 2016, 93, 39–48. [Google Scholar] [CrossRef]
- Spence, C.A.; Lakshmanan, V.; Donofrio, N.; Bais, H.P. Crucial roles of abscisic acid biogenesis in virulence of rice blast fungus Magnaporthe oryzae. Front. Plant Sci. 2015, 6, 1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lievens, L.; Pollier, J.; Goossens, A.; Beyaert, R.; Staal, J. Abscisic acid as pathogen effector and immune regulator. Front. Plant Sci. 2017, 8, 587. [Google Scholar] [CrossRef] [PubMed]
- Buhrow, L.M.; Cram, D.; Tulpan, D.; Foroud, N.A.; Loewen, M.C. Exogenous abscisic acid and gibberellic acid elicit opposing effects on Fusarium graminearum infection in wheat. Phytopathology 2016, 106, 986–996. [Google Scholar] [CrossRef] [Green Version]
- Maksimov, I.V.; Ganiev, R.M.; Khairullin, R.M. Changes in the levels of IAA, ABA, and cytokinins in wheat seedlings infected with Tilletia caries. Russ. J. Plant Physiol. 2002, 49, 221–224. [Google Scholar] [CrossRef]
- Li, X.-Y.; Gao, L.; Zhang, W.-H.; Liu, J.-K.; Zhang, Y.-J.; Wang, H.-Y.; Liu, D.-Q. Characteristic expression of wheat PR5 gene in response to infection by the leaf rust pathogen, Puccinia triticina. J. Plant Interact. 2015, 10, 132–141. [Google Scholar] [CrossRef]
- Edens, L.; Heslinga, L.; Klok, R.; Ledeboer, A.M.; Maat, J.; Toonen, M.Y.; Visser, C.; Verrips, C.T. Cloning of cDNA encoding the sweet-tasting plant protein thaumatin and its expression in Escherichia coli. Gene 1982, 18, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, H.; Wei, X.; Zhang, J.; Wang, H.; Liu, D. Expression analysis and functional characterization of a pathogen-induced thaumatin-like gene in wheat conferring enhanced resistance to Puccinia triticina. J. Plant Interact. 2017, 12, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Dobon, A.; Bunting, D.C.E.; Cabrera-Quio, L.E.; Uauy, C.; Saunders, D.G.O. The host-pathogen interaction between wheat and yellow rust induces temporally coordinated waves of gene expression. BMC Genomics 2016, 17, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, S.; Wenig, M.; Langen, G.; Sharma, S.; Kugler, K.G.; Knappe, C.; Hause, B.; Bichlmeier, M.; Babaeizad, V.; Imani, J.; et al. Bacteria-triggered systemic immunity in barley is associated with WRKY and ethylene responsive factors but not with salicylic acid. Plant Physiol. 2014, 166, 2133–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, S.; Hu, H.; Meinke, H.; Shabala, S.; Westmore, G.; Larkin, P.; Zhou, M. Barley yellow dwarf viruses: Infection mechanisms and breeding strategies. Euphytica 2017, 213, 168. [Google Scholar] [CrossRef]
- Davis, T.S.; Bosque-Pérez, N.A.; Popova, I.; Eigenbrode, S.D. Evidence for additive effects of virus infection and water availability on phytohormone induction in a staple crop. Front. Ecol. Evol. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Paulmann, M.K.; Kunert, G.; Zimmermann, M.R.; Theis, N.; Ludwig, A.; Meichsner, D.; Oelmüller, R.; Gershenzon, J.; Habekuss, A.; Ordon, F.; et al. Barley yellow dwarf virus infection leads to higher chemical defense signals and lower electrophysiological reactions in susceptible compared to tolerant barley genotypes. Front. Plant Sci. 2018, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Shi, J.; Shi, F.; Wang, X.; Xu, H.; He, K.; Wang, Z. Aphid fecundity and defenses in wheat exposed to a combination of heat and drought stress. J. Exp. Bot. 2020, eraa017. [Google Scholar] [CrossRef]
- Marimuthu, M.; Smith, C.M. Barley tolerance of Russian wheat aphid (Hemiptera: Aphididae) biotype 2 herbivory involves expression of defense response and developmental genes. Plant Signal. Behav. 2012, 7, 382–391. [Google Scholar] [CrossRef] [Green Version]



| Gene | Species | Type of Manipulation | Effect | Reference |
|---|---|---|---|---|
| OsABA1 (ZEP) | Rice | Rice Osaba1 mutant | Wilty phenotype; low ABA content even upon drought | [7] |
| TaNCED1 | Wheat | Overexpression of TaNCED1 in tobacco | Improved drought tolerance; increased ABA content; higher rate of relative water and soluble sugars content | [26] |
| HvNCED1 | Barley | Transgenic barley line with downregulation of endogenous HvNCED1 | Reduced ABA level during prolonged drought stress | [27] |
| OsNCED3 | Rice | Overexpression of OsNCED3 in A. thaliana | Improved drought tolerance; increased ABA content; reduced relative water loss | [28] |
| TaABA8′OH1 | Wheat | Overexpression of TaABA8′OH1 in rice | Reduced anther ABA content under cold stress | [29] |
| HvABA8′OH1 | Barley | RNAi silencing of HvABA8′OH1 in barley | Improved drought tolerance; better water use efficiency | [27] |
| OsABA8ox1 (ABA-8′-OH1) | Rice | Overexpression of OsABA8ox1 in rice | Reduced drought and cold tolerance due to low ABA content | [30] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gietler, M.; Fidler, J.; Labudda, M.; Nykiel, M. Abscisic Acid—Enemy or Savior in the Response of Cereals to Abiotic and Biotic Stresses? Int. J. Mol. Sci. 2020, 21, 4607. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21134607
Gietler M, Fidler J, Labudda M, Nykiel M. Abscisic Acid—Enemy or Savior in the Response of Cereals to Abiotic and Biotic Stresses? International Journal of Molecular Sciences. 2020; 21(13):4607. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21134607
Chicago/Turabian StyleGietler, Marta, Justyna Fidler, Mateusz Labudda, and Małgorzata Nykiel. 2020. "Abscisic Acid—Enemy or Savior in the Response of Cereals to Abiotic and Biotic Stresses?" International Journal of Molecular Sciences 21, no. 13: 4607. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21134607