Key Process and Factors Controlling the Direct Translocation of Cell-Penetrating Peptide through Bio-Membrane
Abstract
:1. Introduction
2. Results
2.1. Characterization of GUV Geometry
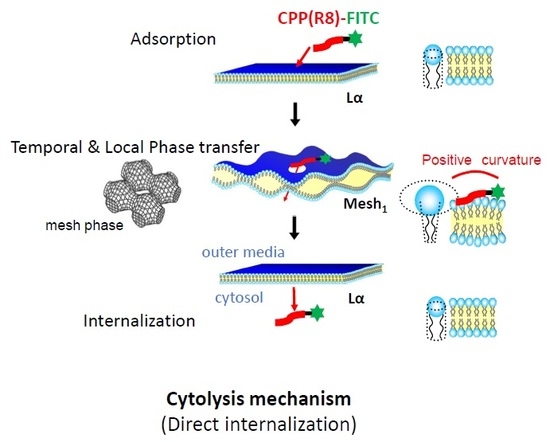
2.2. Cytolysis of CPP to GUV
2.2.1. Distinctive Analysis of Cytolysis to Clarify the CPP Adsorption and Actual Internalization
2.2.2. Time Course Analysis of CPP Translocation to GUV
2.2.3. Effect of Temperature for the CPP Translocation to GUV
2.2.4. Effect of Lipid Composition for the CPP Translocation to GUV
2.2.5. Effect of Solute in the Outer Media for the CPP Translocation to GUV
3. Discussions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of GUV
4.2.2. Confirmation of the GUV Formation
Differential Interference Contrast Microscope (DICM) Observations of GUV
Particle Size Distribution Measurement of GUV
4.2.3. Phospholipid Concentration Measurement
4.2.4. Preparation of Trypsin Solution
4.2.5. Surface Pressure (π)—Molecular Occupation Area (A) Measurement
4.2.6. Calculation of the Number of Phospholipid Molecules Consisting of GUV
4.2.7. Translocation Assay of CPP to GUV
4.2.8. Measurement of Cytolysis Amount of CPP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CPP | Cell-Penetrating Peptide |
| GUV | Giant unilamellar vesicle |
| DICM | differential interference contrast microscope |
| DLS | dynamic light scattering |
References
- Futaki, S.; Nakase, I. Cell-Surface Interactions on Arginine-Rich Cell-Penetrating Peptides Allow for Multiplex Modes of Internalization. Acc. Chem. Res. 2017, 50, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S. Oligoarginine vectors for intracellular delivery: Design and cellular-uptake mechanisms. Biopolymers 2006, 84, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Futaki, S.; Niwa, M.; Tanaka, S.; Ueda, K.; Sugiura, Y. Possible Existence of Common Internalization Mechanizm among Arginine-rich Peptides. J. Biol. Chem. 2002, 277, 2437–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanzel, E.G.; Trantow, B.M.; Vargas, J.R.; Wender, P.A. Fifteen Years of Cell-Penetrating, Guanidinium-Rich Molecular Transporters; Basic Science, Research Tools, and Clinical Applications. Acc. Chem. Res. 2013, 46, 2944–2954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conner, S.D.; Schmid, S.L. Regulated Portals of Entry into the Cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, G.; Kay, R.R. Uses and Abuses of Macropinocytosis. J. Cell. Sci. 2016, 129, 2697–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, J.P.; Melikov, K.; Vives, E.; Ramos, C.; Verbeure, B.; Gait, M.J.; Chernomordik, L.V.; Lebleu, B. Cell-penetrating Peptide—A Reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 2003, 278, 585–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. The molecular geometry is often called as CPP (critical packing parameter), however in this manuscript Surfactant Parameter12 (SP) was employed because CPP is used for Cell-Penetrating Peptide. J. Chem. Soc. Faraday Trans. 1976, 72, 1525–1568. [Google Scholar]
- Hyde, S.; Andersson, S.; Larsson, K.; Blum, Z.; Landh, T.; Linden, S.; Ninham, B.W. The Language of Shape; Elsevier: Amsterdam, The Netherlands, 1997; pp. 143–146. [Google Scholar]
- Sakamoto, K.; Takino, Y.; Ogasahara, K. Method for Controlling Membrane Permeability of a Membrane Permeable Substance and Screening Method for a Membrane Permeable Substance. U.S. Patent 20050118204A1, 4 November 2003. [Google Scholar]
- Sakamoto, K.; Takino, Y.; Ogasahara, K.; Hemayet, U.; Futaki, S. Key Mechanism Triggering Cell-Penetrating Peptide to Cross Membrane. In Proceedings of the 13th IACIS & 83rd ACS Colloid and Surface Sci. Symposium, New York, NY, USA, 19 June 2009. [Google Scholar]
- Sakamoto, K.; Morishita, T.; Aburai, K.; Sakai, K.; Sakai, H.; Abe, M.; Nakase, I.; Futaki, S. Bioinspired Mechanism for the Translocation of Peptide through the Cell Membrane. Chem. Lett. 2012, 41, 1078–1080, This is a Proceedings based on the presentation made at the International Association of Colloid and Interface Scientists, Conference (IACIS2012), Sendai, Japan, 13–18 May 2012. [Google Scholar] [CrossRef]
- Sakamoto, K. The Importance of Planarity for Lipid Bilayers as Biomembrane. In Advances in Biomembranes and Lipid Self-Assembly; Elsevier: Amsterdam, Netherlands, 2016; Volume 23, pp. 1–23. [Google Scholar]
- Hyde, S.T.; Schroder, G.E. Novel surfactant mesostructural topologies: Between lamellae and columnar (hexagonal) forms. Curr. Opin. Colloid Interface Sci. 2003, 8, 5–14. [Google Scholar] [CrossRef]
- Sakamoto, K.; Morishita, T.; Aburai, K.; Ito, D.; Imura, T.; Sakai, K.; Abe, M.; Nakase, I.; Futaki, S.; Sakai, H. Direct entry of Cell-Penetrating Peptide can be controlled by maneuvering the membrane curvature. Sci. Rep. 2020. under review. [Google Scholar]
- Sharmin, S.; Islam, Z.; Karal, M.A.S.; Shibly, S.U.A.; Dohra, H.; Yamazaki, M. Effects of Lipid Composition on the Entry of Cell-Penetrating Peptide Oligoarginine into Single Vesicles. Biochemistry 2016, 55, 4154–4165. [Google Scholar] [PubMed]
- Islam, Z.; Ariyama, H.; Alam, J.M.; Yamazaki, M. Entry of Cell-Penetrating peptide transportan 10 into a single vesicle by translocating across lipid membrane and its induced pores. Biochemistry 2014, 53, 386–396. [Google Scholar] [PubMed]
- Wheaten, S.A.; Ablan, F.D.O.; Spaller, B.L.; Trieu, J.M.; Almeida, P.F. Translocation of Cationic Amphipathic Peptides across the Membranes of Pure Phospholipid Giant Vesicles. J. Am. Chem. Soc. 2013, 135, 16517–16525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuma, Y.; Imai, M. From Vesicles to Protocells: The Roles of Amphiphilic Molecules. Life 2015, 5, 651–675. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, V.; Svetins, S.; Zeks, B. Non asymmetric vesicle shapes. Phys. Rev. E 1993, 48, 3112–3123. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.L.; Seifert, U.; Wortis, M.; Dobereinner, H.G. Budding transitions of fluid-bilayer vesicles: The effect of area-difference elasticity. Phys Rev. E 1994, 49, 5389–5407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, B.; Maestri, D.M.; Perillo, M.A. Phosphatidylcholine/vegetable oil pseudo-binary mixtures at the air–water interface: Predictive formulation of oil blends with selected surface behavior. Colloids Surf. B Biointerfaces 2010, 75, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Dynarowicz-Latka, P.; Hac-Wydro, K. Interactions between phosphatidylcholines and cholesterol in monolayers at the air/water interfaces. Colloids Surf B Biointerfaces 2004, 37, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Stroud, R.M. A Family of Protein-Cutting Proteins. Sci. Am. 1974, 231, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Encapsula’s Nano Science. Available online: http://www.liposomes.org/2009/01/number-of-lipid-molecules-per-liposome.html (accessed on 31 June 2020).









| Parameter | EPC | SOPC | ||
|---|---|---|---|---|
| d | Diameter of liposeme | nm | 1.4 × 104 | 1.4 × 104 |
| h | Thickness of bilayer | nm | 4.7 | 4.7 |
| a | Lipid molecular occupation area | nm2/molecule | 0.71 | 0.84 |
| Ntot | Number of lipids per one liposome | unit | 1.8 × 109 | 1.5 × 109 |
| Nout | Number of outer lipids per one liposome | unit | 8.8 × 108 | 8.8 × 108 |
| Ninter | Number of internal lipids per one liposome | unit | 8.8 × 108 | 8.8 × 108 |
| Nlipo | Number of liposome per 1 mL of liposomal suspension | unit/mL | 5.8 × 108 | 7.0 × 108 |
| PBS Concentration | mM | 7 | 14 | 28 |
|---|---|---|---|---|
| Osmotic pressure | mOsm | 14 | 28 | 56 |
| Zeta potential of GUV without FITC-R8 | mV | −10.6 | −10.8 | −5 |
| Zeta potential of GUV with FITC-R8 | mV | −3.2 | −3.9 | −3.8 |
| PBS Concentration | mM | 7 | 14 | 28 |
|---|---|---|---|---|
| Osmotic pressure | mOsm | 14 | 28 | 56 |
| Rate constant * | µmol/mol EPC/min−1 | 1.1 | 0.99 | 0.96 |
| PBS concentration | mM | 7 | 14 | 28 |
| Temperature (°C) | Phase | FITC-R8 μmol/SOPC mol | ||||
|---|---|---|---|---|---|---|
| Osmotic Pressure (mOsm) | ||||||
| 14 | 28 | 56 | 84 | 112 | ||
| 37 | Liquid crystal | 64 | 65 | 77 | 73 | 67 |
| 4 | Gel | 74 | 81 | 80 | 76 | 74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, K.; Morishita, T.; Aburai, K.; Sakai, K.; Abe, M.; Nakase, I.; Futaki, S.; Sakai, H. Key Process and Factors Controlling the Direct Translocation of Cell-Penetrating Peptide through Bio-Membrane. Int. J. Mol. Sci. 2020, 21, 5466. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155466
Sakamoto K, Morishita T, Aburai K, Sakai K, Abe M, Nakase I, Futaki S, Sakai H. Key Process and Factors Controlling the Direct Translocation of Cell-Penetrating Peptide through Bio-Membrane. International Journal of Molecular Sciences. 2020; 21(15):5466. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155466
Chicago/Turabian StyleSakamoto, Kazutami, Taku Morishita, Kenichi Aburai, Kenichi Sakai, Masahiko Abe, Ikuhiko Nakase, Shiroh Futaki, and Hideki Sakai. 2020. "Key Process and Factors Controlling the Direct Translocation of Cell-Penetrating Peptide through Bio-Membrane" International Journal of Molecular Sciences 21, no. 15: 5466. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155466