Aggregation Behavior, Antibacterial Activity and Biocompatibility of Catanionic Assemblies Based on Amino Acid-Derived Surfactants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catanionic Systems
2.1.1. Surface Tension and Acid-Base Behavior
2.1.2. Fluorescence and Nuclear Magnetic Resonance (NMR)
2.1.3. Dynamic Light Scattering (DLS) and ζ-Potential
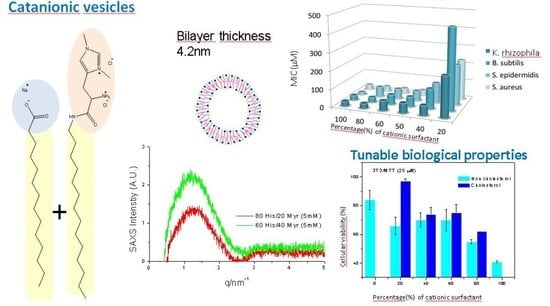
2.1.4. Small-Angle X-Ray Scattering (SAXS)
2.2. Characterization of the Biological Properties
2.2.1. Antibacterial Activity
2.2.2. Hemolytic Activity
2.2.3. Cytotoxicity
3. Materials and Methods
3.1. Materials
3.2. Catanionic Formulations
3.3. Surface Tension
3.4. Fluorescence Measurements
3.5. NMR Measurements
3.6. ζ-potential and size distribution analysis
3.7. pKa Determination
3.8. Small-Angle X-Ray Scattering
3.9. Antibacterial Activity
3.10. Hemolysis assay and Cytotoxicity
3.10.1. Hemolysis Determination
3.10.2. Cell Cultures
3.10.3. Cell Viability Assays
3.10.4. MTT Assay
3.10.5. NRU Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jurasin, D.D.; Segota, S.; Cadez, V.; Selmani, A.; Sikirc, M.D. Recent Advances in Catanionic Mixtures. In Applications and Characterization of Surfactants; Najjar, R., Ed.; IntechOpen: London, UK, 2017; ISBN 978-953-51-3326-1. [Google Scholar]
- Dhawan, V.V.; Nagarsenker, M.S. Catanionic systems in nanotherapeutics-Biophysical aspect and novel trends in drug delivery applications. J. Control. Release 2017, 266, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves Lopes, R.C.F.; Oscar, F.S.; Faria, A.R.; do Vale, M.L.C.; Marques, E.F.; Nieder, J.B. Surface charge tunable catanionic vesicles based on serine-derived surfactants as efficient nanocarriers for the delivery of the anticancer drug doxorubicin. Nanoscale 2019, 11, 5932–5941. [Google Scholar] [CrossRef] [PubMed]
- Tondre, C.; Caillet, C. Properties of the amphiphilic films in mixed cationic/anionic vesicles: A comprehensive view from a literature analysis. Adv. Colloid Interface Sci. 2001, 93, 115–134. [Google Scholar] [CrossRef]
- Ferreira, A.; Mikhailovskaya, A.; Chenneviere, F.; Restagno, F.; Cousin, F.; Muller, J.; Degrouard, A.; Salonen, A.; Marques, E.F. Interplay between bulk self-assembly, interfacial and foaming properties in a catanionic surfactant mixture of varying composition. Soft Matter 2017, 13, 7197–7206. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Qiao, W.; Zongshi, L. Vesicles from pH-regulated reversible gemini amino-acid surfactants as nanocapsules for delivery. Colloids Surf. B 2016, 146, 523–531. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Wang, Y.; Li, X. CO2-Switchable Viscoelastic Fluids Based on a Pseudogemini Surfactant. Langmuir 2013, 29, 4187–4192. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, N.; Wang, Z.; Zhang, X. Photocontrolled reversible supramolecular assemblies of an azobenzene-containing surfactant with alpha-cyclodextrin. Angew. Chem. Int. Ed. 2007, 46, 2823–2826. [Google Scholar] [CrossRef]
- Yoshimura, T.; Chiba, N.; Matsuoka, K. Supra-long chain surfactants with double or triple quaternary ammonium headgroups. J. Colloid Interface Sci. 2012, 374, 157–163. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Haldar, J. Thermodynamics of Micellization of Multiheaded Single-Chain Cationic Surfactants. Langmuir 2004, 20, 7940–7947. [Google Scholar] [CrossRef]
- Hagslätt, H.; Söderman, O.; Jönsson, B.; Johansson, L.B.A. Divalent surfactants. General characterization of the dodecyl-1,3-propylene-Bis(ammonium chloride)/water system. J. Phys. Chem. 1991, 95, 1703–1710. [Google Scholar] [CrossRef]
- Ghosh, S.; Ray, A.; Pramanik, N.; Ambade, B. Can a cationic surfactant mixture act as a drug delivery vehicle? Comptes Rendus Chim. 2016, 19, 951–954. [Google Scholar] [CrossRef]
- Lin, Y.; Han, X.; Cheng, X.; Huang, J.; Liang, D.; Yu, C. pH-regulated molecular self-assemblies in a cationic-anionic surfactant system: From a “1-2” surfactant pair to a “1-1” surfactant pair. Langmuir 2008, 24, 13918–13924. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, A.; Pons, R.; Bustelo, M.; Manresa, M.A.; Moran, C.; Raluy, M.; Perez, L. Gemini histidine based surfactants: Characterization; surface properties and biological activity. J. Mol. Liq. 2019, 289. [Google Scholar] [CrossRef]
- Bustelo, M.; Pinazo, A.; Manresa, M.A.; Mitjans, M.; Vinardell, M.P.; Pérez, L. Monocatenary histidine-based surfactants: Role of the alkyl chain length in antimicrobial activity and their selectivity over red blood cells. Colloids Surf. A 2017, 532, 501–509. [Google Scholar] [CrossRef]
- Martinez-Negro, M.; Blanco-Fernandez, L.; Tentori, P.M.; Perez, L.; Pinazo, A.; de Ilarduya, C.T.; Aicart, E.; Junquera, E. A Gemini Cationic Lipid with Histidine Residues as a Novel Lipid-Based Gene Nanocarrier: A Biophysical and Biochemical Study. Nanomaterials 2018, 8, 1061. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Arribas, N.; Martínez-Negro, M.; Villar, E.M.; Pérez, L.; Osío Barcina, J.; Aicart, E.; Taboada, P.; Guerrero-Martínez, A.; Junquera, E. Protein Expression Knockdown in Cancer Cells Induced by a Gemini Cationic Lipid Nanovector with Histidine-Based Polar Heads. Pharmaceutics 2020, 12, 791. [Google Scholar] [CrossRef]
- Shome, A.; Kar, T.; Das, P.K. Spontaneous Formation of Biocompatible Vesicles in Aqueous Mixtures of Amino Acid-Based Cationic Surfactants and SDS/SDBS. ChemPhysChem 2011, 12, 369–378. [Google Scholar] [CrossRef]
- Liang, C.H.; Yeh, L.H.; Liao, P.W.; Choua, T.H. Characterization and in vitro biocompatibility of catanionic assemblies formed with oppositely charged dicetyl amphiphiles. Colloids Surf. B 2015, 126, 10–17. [Google Scholar] [CrossRef]
- Kuo, J.-h.S.; Chang, C.-H.; Lin, Y.-L.; Wu, C.-J. Flow cytometric characterization of interactions between U-937 human macrophages and positively charged catanionic vesicles. Colloid Surf. B 2008, 64, 307–313. [Google Scholar] [CrossRef]
- Xu, L.; Feng, L.; Dong, R.; Hao, J.; Dong, S. Transfection Efficiency of DNA Enhanced by Association with Salt-Free Catanionic Vesicles. Biomacromolecules 2013, 14, 2781–2789. [Google Scholar] [CrossRef]
- Li, P.X.; Thomas, R.K.; Penfold, J. Limitations in the Use of Surface Tension and the Gibbs Equation to Determine Surface Excesses of Cationic Surfactants. Langmuir 2014, 30, 6739–6747. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.M.; Rubingh, D.N. Nonideal multicomponent mixed micelle model. J. Phys. Chem. 1983, 87, 1984–1990. [Google Scholar] [CrossRef]
- Kralchevsky, P.A.; Danov, K.D.; Pishmanova, C.I.; Kralchevska, S.D.; Christov, N.C.; Ananthapadmanabhan, K.P.; Lips, A. Effect of the Precipitation of Neutral-Soap, Acid-Soap, and Alkanoic Acid Crystallites on the Bulk pH and Surface Tension of Soap Solutions. Langmuir 2007, 23, 3538–3553. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Franses, E.I. Effect of protonation on the solution and phase behavior of aqueous sodium myristate. J. Colloid Interface Sci. 2000, 231, 42–51. [Google Scholar] [CrossRef]
- Aguiar, J.; Carpena, P.; Molina-Bolívar, J.A.; Carnero Ruiz, C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Lozano, N.; Pinazo, A.; La Mesa, C.; Perez, L.; Andreozzi, P.; Pons, R. Catanionic Vesicles Formed with Arginine-Based Surfactants and 1,2-Dipalmitoyl-sn-glycero-3-phosphate Monosodium Salt. J. Phys. Chem. B 2009, 113, 6321–6327. [Google Scholar] [CrossRef]
- Pérez, L.; Pinazo, A.; Infante, M.R.; Pons, R. Investigation of the Micellization Process of Single and Gemini Surfactants from Arginine by SAXS, NMR Self-Diffusion, and Light Scattering. J. Phys. Chem. B 2007, 111, 11379–11387. [Google Scholar] [CrossRef]
- Silva, S.G.; do Vale, M.L.C.; Marques, E.F. Size, Charge, and Stability of Fully Serine-Based Catanionic Vesicles: Towards Versatile Biocompatible Nanocarriers. Chem.-Eur. J. 2015, 21, 4092–4101. [Google Scholar] [CrossRef]
- Diz, M.; Manresa, A.; Pinazo, A.; Erra, P.; Infante, M.R. Synthesis, Surface Active Properties and Antimicrobial Activity of New Bis Quaternary Ammonium Compounds. J. Chem. Soc. Perkin Trans. 1994, 2, 1871–1876. [Google Scholar] [CrossRef]
- Pinazo, A.; Manresa, M.A.; Marques, A.M.; Bustelo, M.; Espuny, M.J.; Perez, L. Amino Acid-based Surfactants: New Antimicrobial Agents. Adv. Colloid Interface Sci. 2016, 228, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Uppu, D.S.S.M.; Haldar, J. Lipopolysaccharide Neutralization by Cationic-Amphiphilic Polymers through Pseudo aggregate Formation. Biomacromolecules 2016, 17, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, A.; Pons, R.; Marqués, A.; Farfan, M.; da Silva, A.; Perez, L. Biocompatible Catanionic Vesicles from Arginine-Based Surfactants: A New Strategy to Tune the Antimicrobial Activity and Cytotoxicity of Vesicular Systems. Pharmaceutics 2020, 12, 857. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, A.; Pérez, L.; Infante, M.R.; Vinardell, M.P.; Mitjans, M.; Morán, M.C.; Martinez, V. Chemical structure and toxicity in arginine-based surfactants. In Arginine Amino Acid; Jacobs, N.L., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; ISBN 978-1-61761-981-6. [Google Scholar]
- Lozano, N.; Perez, L.; Pons, R.; Pinazo, A. Diacyl glycerol arginine-based surfactants: Biological and physicochemical properties of catanionic formulations. Amino Acids 2011, 40, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Chia-Hua, L.; Wen-Yueh, H.; Li-Hsien, Y.; Yu-Shen, C.; Tzung-Han, C. Effects of 1-hexadecyl-3-methylimidazolium ionic liquids on the physicochemical characteristics and cytotoxicity of phosphatidylcholine vesicles. Colloids Surf. A 2013, 436, 1083–1091. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, C.F.; Hsieh, Y.L.; Wang, C.W.; Yang, T.Y.; Chang, C.H.; Yang, Y.M. Effects of Ethanol and Cholesterol on Thermotropic Phase Behavior of Ion-Pair Amphiphile Bilayers. J. Oleo Sci. 2018, 67, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Cortesi, R.; Esposito, E.; Menegatti, E.; Gambari, R.; Nastruzzi, C. Effect of cationic liposome composition on in vitro cytotoxicity and protective effect on carried DNA. Int. J. Pharm. 1996, 139, 69–78. [Google Scholar] [CrossRef]
- Nogueira, D.R.; Morán, M.C.; Mitjans, M.; Martínez, V.; Pérez, L. New cationic nanovesicular systems containing lysine-based surfactants for topical administration: Toxicity assessment using representative skin cell lines. Eur. J. Pharm. Biopharm. 2013, 83, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Repetto, G.; Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insight into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [CrossRef]
- Nogueira, D.R.; Mitjans, M.; Infante, M.R.; Vinardell, M.P. Comparative sensitivity of tumor and non-tumor cell lines as a reliable approach for in vitro cytotoxicity screening of lysine-based surfactants with potential pharmaceutical applications. Int. J. Pharm. 2011, 420, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonez, C.; Vandenbranden, M.; Ruysschaert, J.-M. Cationic lipids activate intracellular signaling pathways. Adv. Drug Deliv. Rev. 2012, 64, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-H.; Chou, T.-H. Effect of chain length on physicochemical properties and cytotoxicity of cationic vesicles composed of phosphatidylcholines and dialkyldimethylammonium bromides. Chem. Phys. Lipids 2009, 158, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, Y.; Takano, S.; Tsuchiya, S. Induction of apoptosis in macrophages by cationic liposomes. FEBS Lett. 1999, 460, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.R.; Bondurant, B.; McLean, S.D.; McGovern, K.A.; O’Brien, D.F. Liposome-cell interactions in vitro: Effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry 1998, 37, 12875–12883. [Google Scholar] [CrossRef]
- Tavano, L.; Infante, M.R.; Abo Riya, M.; Pinazo, A.; Vinardell, M.P.; Mitjans, M.; Manresa, M.A.; Perez, L. Role of aggregate size in the hemolytic and antimicrobial activity of colloidal solutions based on single and gemini surfactants from arginine. Soft Matter 2013, 9, 306–319. [Google Scholar] [CrossRef]
- Sorrenti, A.; Illa, O.; Pons, R.; Ortuño, R.M. Chiral Cyclobutane β-Amino Acid-Based Amphiphiles: Influence of cis/trans Stereochemistry on Solution Self-Aggregation and Recognition. Langmuir 2015, 31, 9608–9618. [Google Scholar] [CrossRef] [Green Version]
- Haba, E.; Pinazo, A.; Pons, R.; Pérez, L.; Manresa, A. Complex rhamnolipid mixture characterization and its influence on DPPC bilayer organization. Biochim. Biophys. Acta Biomembr. 2014, 1838, 776–783. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.B.; Tenover, F.C.; Turnidge, J.D.; Jorgensen, J.H. Manual of Clinical Microbiology, 10th ed.; Versalovic, J., Carrol, K.C., Funke, G., Jorgensen, J.H., Landry, M.L., Warnock, D.W., Eds.; ASM Press: Washinton, DC, USA, 2011. [Google Scholar]
- Pape, W.J.; Pfannenbecker, U.; Hoppe, U. Validation of the Red Blood Cell Test System as in Vitro Assay for the Rapid Screening of Irritation Potential of Surfactants. Mol. Toxicol. 1987, 1, 525–536. [Google Scholar]




 80:20 HM,
80:20 HM,  , 60:40 HM,
, 60:40 HM,  50:50 HM,
50:50 HM,  40:60 HM,
40:60 HM,  20:80 HM. (B) mean DLS diameter (
20:80 HM. (B) mean DLS diameter (  left) and ζ-potential results (
left) and ζ-potential results (  right) for the HM formulations.
right) for the HM formulations.
 80:20 HM,
80:20 HM,  , 60:40 HM,
, 60:40 HM,  50:50 HM,
50:50 HM,  40:60 HM,
40:60 HM,  20:80 HM. (B) mean DLS diameter (
20:80 HM. (B) mean DLS diameter (  left) and ζ-potential results (
left) and ζ-potential results (  right) for the HM formulations.
right) for the HM formulations.


 Histidine,
Histidine,  Sodium myristate,
Sodium myristate,  80:20 HM,
80:20 HM,  80:20 HM_COL,
80:20 HM_COL,  60:40 HM,
60:40 HM,  60:40 HM_COL,
60:40 HM_COL,  40:60 HM,
40:60 HM,  40:60 HM_COL,
40:60 HM_COL,  20:80 HM,
20:80 HM,  20:80 HM_COL.
20:80 HM_COL.
 Histidine,
Histidine,  Sodium myristate,
Sodium myristate,  80:20 HM,
80:20 HM,  80:20 HM_COL,
80:20 HM_COL,  60:40 HM,
60:40 HM,  60:40 HM_COL,
60:40 HM_COL,  40:60 HM,
40:60 HM,  40:60 HM_COL,
40:60 HM_COL,  20:80 HM,
20:80 HM,  20:80 HM_COL.
20:80 HM_COL.
 Histidine,
Histidine,  Sodium myristate,
Sodium myristate,  80:20 HM,
80:20 HM,  80:20 HM_COL,
80:20 HM_COL,  60:40 HM,
60:40 HM,  60:40 HM_COL,
60:40 HM_COL,  40:60 HM,
40:60 HM,  40:60 HM_COL,
40:60 HM_COL,  20:80 HM,
20:80 HM,  20:80 HM_COL.
20:80 HM_COL.
 Histidine,
Histidine,  Sodium myristate,
Sodium myristate,  80:20 HM,
80:20 HM,  80:20 HM_COL,
80:20 HM_COL,  60:40 HM,
60:40 HM,  60:40 HM_COL,
60:40 HM_COL,  40:60 HM,
40:60 HM,  40:60 HM_COL,
40:60 HM_COL,  20:80 HM,
20:80 HM,  20:80 HM_COL.
20:80 HM_COL.
| DMHNHC14 (%) | Sodium Myristate (%) | DMHNHC14 (%) | C12C3L (%) | ||
|---|---|---|---|---|---|
| 80:20 HM | 80 | 20 | 80:20 HL | 80 | 20 |
| 60:40 HM | 60 | 40 | 60:40 HL | 60 | 40 |
| 50:50 HM 40:60 HM 20:80 HM | 50 40 20 | 50 60 80 | 40:60 HL 20:80 HL | 40 20 | 60 80 |
| Mixtures with Cholesterol | |||||
| Cholesterol (%) | DMHNHC14 (%) | Sodium Myristate (%) | |||
| 80:20 HM_COL | 20 | 64 | 16 | ||
| 60:40 HM_COL | 20 | 48 | 32 | ||
| 40:60 HM_COL | 20 | 32 | 48 | ||
| 20:80 HM_COL | 20 | 16 | 64 | ||
| HM | Am 1 | CMC γ 2 | γ CMC 3 | α 4 | β 5 | CMCF 2 | α 4 | β 5 | ζ-potential 6 |
|---|---|---|---|---|---|---|---|---|---|
| 100 | 0.57 | 5.2 | 33 | 3.8 | |||||
| 80:20 HM | 0.28 | 0.17 | 23.5 | 0.49 | −11.3 | 0.60 | 0.49 | −5.5 | +45 |
| 60:40 HM | 0.26 | 0.10 | 23 | 0.458 | −12.5 | 0.47 | 0.43 | −5.4 | +43 |
| 50:50 HM | 0.17 | 0.096 | 23 | 0.44 | −12.3 | 0.29 | 0.41 | −7.3 | +36 |
| 40:60 HM | 0.22 | 0.090 | 22 | 0.43 | −12.6 | 0.31 | 0.39 | −6.7 | +33 |
| 20:80 HM | 0.20 | 0.12 | 22 | 0.39 | −11.9 | 0.40 | 0.32 | −5.6 | −24 |
| Myr | 1.0 | 5.0 7 | 24 | 0.90 |
| MIC (μM) | ||||||
|---|---|---|---|---|---|---|
| DMHNHC14 | 80:20 HM | 60:40 HM | 50:50 HM | 40:60 HM | 20:80 HM | |
| Kocuria rhizophila ATCC 9341 | 28 | 28 (22) | 56 (34) | 56 (28) | 56 (22) | 227 (45) |
| Bacillus subtilis ATCC 6633 | 28 | 28 (22) | 56 (34) | 56 (28) | 113 (45) | 454 (90) |
| Staphylococcus epidermidis ATCC 12228 | 14 | 56 (45) | 56 (34) | 28 (14) | 113 (45) | 227 (45) |
| Staphylococcus aureus ATCC 29213 | 28 | 28 (22) | 28 (17) | 28 (14) | 56 (45) | 227 (45) |
| Klebsiella pneumoniae ATCC 13883 | 113 | 227 (181) | 227 (136) | > (>227) | > (>181) | > (>90) |
| Escherichia coli ATCC 25922 | 113 | 113 (90) | 227 (136) | >454 (>227) | >454 (>181) | >454 (>90) |
| Pseudomonas aeruginosa ATCC 27853 | 227 | >454 (>363) | >454 (>271) | >454 (>227) | >454 (>181) | >454 (>90) |
| Candida albicans ATCC 10231 | 28 | 28 (22) | 56 (34) | 56 (28) | 56 (22) | 113 (23) |
| MIC (μM) | |||||
|---|---|---|---|---|---|
| DMHNHC14 | 80:20 HL | 60:40 HL | 40:60 HL | 20:80 HL | |
| Kocuria rhizophila ATCC 9341 | 28 | 28 (22) | 28 (17) | 56 (22) | 56 (11) |
| Bacillus subtilis ATCC 6633 | 28 | 113 (90) | 113 (68) | 113 (45) | 113 (23) |
| Staphylococcus epidermidis ATCC 12228 | 14 | 113 (90) | 113 (68) | 227 (90) | 454 (90) |
| Staphylococcus aureus ATCC 29213 | 28 | 113 (90) | 113 (68) | 113 (45) | 227 (45) |
| Klebsiella pneumoniae ATCC 13883 | 113 | 227 (181) | 454 (272) | >454 (>181) | >454 (>90) |
| Escherichia coli ATCC 25922 | 113 | 113 (90) | 113 (68) | 227 (90) | >454 (>90) |
| Pseudomonas aeruginosa ATCC 27853 | 227 | >454 (>363) | >454 (>271) | >454 (>181) | >454 (>90) |
| Candida albicans ATCC 10231 | 28 | 56 (45) | 113 (68) | 113 (45) | 113 (23) |
| SYSTEM | Cellular Line | |||||||
|---|---|---|---|---|---|---|---|---|
| 3T3 | HaCaT | HeLa | A431 | |||||
| MTT | NRU | MTT | NRU | MTT | NRU | MTT | NRU | |
| DMHNHC14 | 17.1 | 26.1 | 25.5 | 45.7 | 3.37 | >50 | 22.6 | >50 |
| Myristate | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| 80:20 HM | 34.1 | >50 | 15.5 | >50 | 38.3 | >50 | 29.5 | 26.9 |
| 80:20 HM_COL | >50 | >50 | 21.8 | >50 | 44.5 | >50 | >50 | >50 |
| 60:40 HM | 34.8 | 36.5 | >50 | >50 | >50 | >50 | >50 | 36.1 |
| 60:40 HM_COL | >50 | 39.9 | >50 | >50 | >50 | >50 | >50 | >50 |
| 40:60 HM | 50 | 38.3 | >50 | >50 | >50 | >50 | >50 | >50 |
| 40:60 HM_COL | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| 20:80 HM | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| 20:80 HM_COL | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, L.; Pinazo, A.; Morán, M.C.; Pons, R. Aggregation Behavior, Antibacterial Activity and Biocompatibility of Catanionic Assemblies Based on Amino Acid-Derived Surfactants. Int. J. Mol. Sci. 2020, 21, 8912. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21238912
Pérez L, Pinazo A, Morán MC, Pons R. Aggregation Behavior, Antibacterial Activity and Biocompatibility of Catanionic Assemblies Based on Amino Acid-Derived Surfactants. International Journal of Molecular Sciences. 2020; 21(23):8912. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21238912
Chicago/Turabian StylePérez, Lourdes, Aurora Pinazo, M. C. Morán, and Ramon Pons. 2020. "Aggregation Behavior, Antibacterial Activity and Biocompatibility of Catanionic Assemblies Based on Amino Acid-Derived Surfactants" International Journal of Molecular Sciences 21, no. 23: 8912. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21238912