Bisphosphonates Reduce Smoking-Induced Osteoporotic-Like Alterations by Regulating RANKL/OPG in an Osteoblast and Osteoclast Co-Culture Model
Abstract
:1. Introduction
2. Results
2.1. Co-Cultures of Osteoblasts and Osteoclasts Were More Stable than Mono-Cultures, and Co-Cultures Showed More Pronounced Effects from the Investigated Substances than Mono-Cultures Did
2.2. CSE Had a Dose-Dependent Negative Effect on Cell Viability in Co-Cultures of Osteoblasts and Osteoclasts
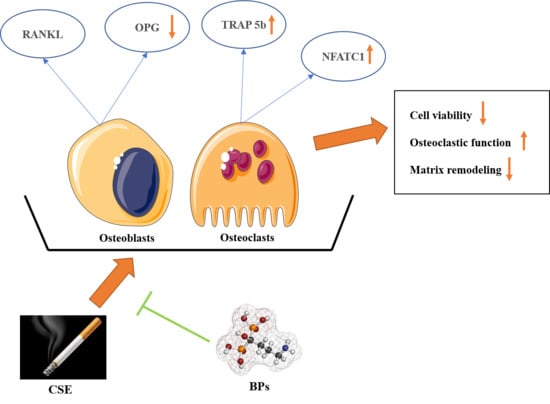
2.3. CSE Induced Osteoporotic-Like Alterations in Co-Cultures of Osteoblasts and Osteoclasts by Up-Regulating Osteoclastic Function
2.4. BPs (Zoledronate and Alendronate) Counteracted the Effects of CSE on Co-Cultures of SaOS-2 and THP-1 Cells
2.5. CSE Exposure Enhanced Gene Expression of Osteoclastic Markers by Increasing the RANKL/OPG Raio, and BPs May Conteract the Effects of CSE on Co-Cultures
2.6. BPs Conteracted the Effects of CSE on Elevating Protein Expression of Osteoclastic Markers by Increasing the RANKL/OPG Ratio in Co-Cultures
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.1.1. Culture of Cell Lines
4.1.2. Cell Seeding
4.1.3. Osteogenic Differentiation
4.2. Generation of CSE
4.3. Sulforhodamine B (SRB) Staining
4.4. Actin and Nuclei Staining
4.5. Carbonic Anhydrate II (CA II) Assay
4.6. TRAP 5b Activity
4.7. Alizarin Red Staining
4.8. Gene Expression Analysis
4.9. Protein Level Analysis
4.10. Total DNA Isolation and Quantification
4.11. Cell-Type-Specific DNA Quantification for Co-Cultures
4.12. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BPs | Bisphosphonates |
| CA II | Carbonic anhydrate II |
| cDNA | Complementary DNA |
| CS | Cigarette smoking |
| CSE | Cigarette smoke extract |
| DEPC | Diethyl pyrocarbonate |
| ECL | Enhanced Chemiluminescence |
| EDTA | Ethylenediaminetetraacetic acid |
| MCS-F | Macrophage colony-stimulating factor |
| NFATC1 | Nuclear factor of activated T cells 1 |
| OPG | Osteoprotegerin |
| PMA | Phorbol-12-myristate 13-acetate |
| RANKL | Receptor activator of nuclear factor (NF)-kB-ligand |
| SaOS-2 | Human osteosarcoma cell line |
| THP-1 | Human monocytic leukemia cell line |
| TRAP | Tartrate-resistant acid phosphatase |
| TRITC | Tetramethylrhodamine |
| SRB | Sulforhodamine B |
| SRY | Sex-determining region Y |
References
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sieberath, A.; Della Bella, E.; Ferreira, A.M.; Gentile, P.; Eglin, D.; Dalgarno, K. A Comparison of Osteoblast and Osteoclast In Vitro Co-Culture Models and Their Translation for Preclinical Drug Testing Applications. Int. J. Mol. Sci. 2020, 21, 912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemann, C.; Heinemann, S.; Rossler, S.; Kruppke, B.; Wiesmann, H.P.; Hanke, T. Organically modified hydroxyapatite (ormoHAP) nanospheres stimulate the differentiation of osteoblast and osteoclast precursors: A co-culture study. Biomed. Mater. 2019, 14, 035015. [Google Scholar] [CrossRef]
- Zhu, S.; Ehnert, S.; Rouss, M.; Haussling, V.; Aspera-Werz, R.H.; Chen, T.; Nussler, A.K. From the Clinical Problem to the Basic Research-Co-Culture Models of Osteoblasts and Osteoclasts. Int. J. Mol. Sci. 2018, 19, 2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, G.S.; Li, Y.; Zhao, G.; Zhou, H.B.; Xie, Z.G.; Xu, W.; Chen, H.N.; Dong, Q.R.; Xu, Y.J. Cigarette smoking and risk of hip fracture in women: A meta-analysis of prospective cohort studies. Injury 2015, 46, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.J.; Zhao, P.; Liu, B.; Yuan, Z.C. Effect of Cigarette Smoking on Risk of Hip Fracture in Men: A Meta-Analysis of 14 Prospective Cohort Studies. PLoS ONE 2016, 11, e0168990. [Google Scholar] [CrossRef]
- Aspera-Werz, R.H.; Chen, T.; Ehnert, S.; Zhu, S.; Frohlich, T.; Nussler, A.K. Cigarette Smoke Induces the Risk of Metabolic Bone Diseases: Transforming Growth Factor Beta Signaling Impairment via Dysfunctional Primary Cilia Affects Migration, Proliferation, and Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 2915. [Google Scholar] [CrossRef] [Green Version]
- Aspera-Werz, R.H.; Ehnert, S.; Heid, D.; Zhu, S.; Chen, T.; Braun, B.; Sreekumar, V.; Arnscheidt, C.; Nussler, A.K. Nicotine and Cotinine Inhibit Catalase and Glutathione Reductase Activity Contributing to the Impaired Osteogenesis of SCP-1 Cells Exposed to Cigarette Smoke. Oxid. Med. Cell. Longev. 2018, 2018, 3172480. [Google Scholar] [CrossRef] [Green Version]
- Reumann, M.K.; Schaefer, J.; Titz, B.; Aspera-Werz, R.H.; Wong, E.T.; Szostak, J.; Haussling, V.; Ehnert, S.; Leroy, P.; Tan, W.T.; et al. E-vapor aerosols do not compromise bone integrity relative to cigarette smoke after 6-month inhalation in an ApoE(-/-) mouse model. Arch. Toxicol. 2020, 94, 2163–2177. [Google Scholar] [CrossRef]
- Park, E.; Kim, J.; Kim, M.C.; Yeo, S.; Kim, J.; Park, S.; Jo, M.; Choi, C.W.; Jin, H.S.; Lee, S.W.; et al. Anti-Osteoporotic Effects of Kukoamine B Isolated from Lycii Radicis Cortex Extract on Osteoblast and Osteoclast Cells and Ovariectomized Osteoporosis Model Mice. Int. J. Mol. Sci. 2019, 20, 2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, V.; Maalouf, N.M.; Sakhaee, K. The effects of smoking on bone metabolism. Osteoporos. Int. 2012, 23, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Marinucci, L.; Balloni, S.; Fettucciari, K.; Bodo, M.; Talesa, V.N.; Antognelli, C. Nicotine induces apoptosis in human osteoblasts via a novel mechanism driven by H2O2 and entailing Glyoxalase 1-dependent MG-H1 accumulation leading to TG2-mediated NF-kB desensitization: Implication for smokers-related osteoporosis. Free Radic Biol. Med. 2018, 117, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Strozyk, D.; Gress, T.M.; Breitling, L.P. Smoking and bone mineral density: Comprehensive analyses of the third National Health and Nutrition Examination Survey (NHANES III). Arch. Osteoporos. 2018, 13, 16. [Google Scholar] [CrossRef]
- Kolkesen Sahin, O.; Cina Aksoy, M.; Avunduk, M.C. Effects of resveratrol and cigarette smoking onbone healing: Histomorphometric evaluation. Turk J. Med. Sci. 2016, 46, 1203–1208. [Google Scholar] [CrossRef]
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef]
- Acil, Y.; Arndt, M.L.; Gulses, A.; Wieker, H.; Naujokat, H.; Ayna, M.; Wiltfang, J. Cytotoxic and inflammatory effects of alendronate and zolendronate on human osteoblasts, gingival fibroblasts and osteosarcoma cells. J. Craniomaxillofac. Surg. 2018, 46, 538–546. [Google Scholar] [CrossRef]
- Lehenkari, P.; Hentunen, T.A.; Laitala-Leinonen, T.; Tuukkanen, J.; Vaananen, H.K. Carbonic anhydrase II plays a major role in osteoclast differentiation and bone resorption by effecting the steady state intracellular pH and Ca2+. Exp. Cell Res. 1998, 242, 128–137. [Google Scholar] [CrossRef]
- Jeong, J.W.; Choi, S.H.; Han, M.H.; Kim, G.Y.; Park, C.; Hong, S.H.; Lee, B.J.; Park, E.K.; Kim, S.O.; Leem, S.H.; et al. Protective Effects of Fermented Oyster Extract against RANKL-Induced Osteoclastogenesis through Scavenging ROS Generation in RAW 264.7 Cells. Int. J. Mol. Sci. 2019, 20, 1439. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Ebetino, F.H.; Hogan, A.M.; Sun, S.; Tsoumpra, M.K.; Duan, X.; Triffitt, J.T.; Kwaasi, A.A.; Dunford, J.E.; Barnett, B.L.; Oppermann, U.; et al. The relationship between the chemistry and biological activity of the bisphosphonates. Bone 2011, 49, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.P.; Yekta, S.S.; Merkel, C.; Ziebart, T.; Smeets, R. The impact of bisphosphonates on the osteoblast proliferation and Collagen gene expression in vitro. Head Face Med. 2010, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Dong, Y.; Huang, X.; Chen, P.; Guo, F.; Chen, A.; Huang, S. Pioglitazone affects the OPG/RANKL/RANK system and increase osteoclastogenesis. Mol. Med. Rep. 2016, 14, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- de Castro, L.F.; Burke, A.B.; Wang, H.D.; Tsai, J.; Florenzano, P.; Pan, K.S.; Bhattacharyya, N.; Boyce, A.M.; Gafni, R.I.; Molinolo, A.A.; et al. Activation of RANK/RANKL/OPG Pathway Is Involved in the Pathophysiology of Fibrous Dysplasia and Associated With Disease Burden. J. Bone Miner. Res. 2019, 34, 290–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, R.; Reilly, G.C. In vitro Models of Bone Remodelling and Associated Disorders. Front. Bioeng. Biotechnol 2018, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, B.; Pitsillides, A.A. Aging and Mechanoadaptive Responsiveness of Bone. Curr. Osteoporos. Rep. 2019, 17, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roeder, I.; Loeffler, M.; Glauche, I. Towards a quantitative understanding of stem cell-niche interaction: Experiments, models, and technologies. Blood Cells Mol. Dis. 2011, 46, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Swanson, M.H.; Enderby, L.T.; D’Amico, F.; Enderby, B.; Samsonraj, R.M.; Dudakovic, A.; van Wijnen, A.J.; Witt-Enderby, P.A. Melatonin-micronutrients Osteopenia Treatment Study (MOTS): A translational study assessing melatonin, strontium (citrate), vitamin D3 and vitamin K2 (MK7) on bone density, bone marker turnover and health related quality of life in postmenopausal osteopenic women following a one-year double-blind RCT and on osteoblast-osteoclast co-cultures. Aging 2017, 9, 256–285. [Google Scholar] [CrossRef] [Green Version]
- Schulze, S.; Wehrum, D.; Dieter, P.; Hempel, U. A supplement-free osteoclast-osteoblast co-culture for pre-clinical application. J. Cell. Physiol. 2018, 233, 4391–4400. [Google Scholar] [CrossRef]
- Li, Z.H.; Si, Y.; Xu, G.; Chen, X.M.; Xiong, H.; Lai, L.; Zheng, Y.Q.; Zhang, Z.G. High-dose PMA with RANKL and MCSF induces THP1 cell differentiation into human functional osteoclasts in vitro. Mol. Med. Rep. 2017, 16, 8380–8384. [Google Scholar] [CrossRef] [Green Version]
- Ehnert, S.; Linnemann, C.; Aspera-Werz, R.H.; Häussling, V.; Braun, B.; Weng, W.; Zhu, S.; Ngamsri, K.C.; Nussler, A. Feasibility of Cell Lines for In Vitro Co-CultuRes. Models for Bone Metabolism. SciMed. J. 2020, 2, 157–181. [Google Scholar] [CrossRef]
- Hamdy, R.C.; Dickerson, K.; Whalen, K. Cigarette Smoking and Bone Health. J. Clin. Densitom. 2020. [Google Scholar] [CrossRef]
- Bon, J.; Kahloon, R.; Zhang, Y.; Xue, J.; Fuhrman, C.R.; Tan, J.; Burger, M.; Kass, D.J.; Csizmadia, E.; Otterbein, L.; et al. Autoreactivity to glucose regulated protein 78 links emphysema and osteoporosis in smokers. PLoS ONE 2014, 9, e105066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeam, C.T.; Chia, S.; Tan, H.C.C.; Kwan, Y.H.; Fong, W.; Seng, J.J.B. A systematic review of factors affecting medication adherence among patients with osteoporosis. Osteoporos. Int. 2018, 29, 2623–2637. [Google Scholar] [CrossRef] [PubMed]
- Habermann, B.; Eberhardt, C.; Feld, M.; Zichner, L.; Kurth, A.A. Tartrate-resistant acid phosphatase 5b (TRAP 5b) as a marker of osteoclast activity in the early phase after cementless total hip replacement. Acta Orthop. 2007, 78, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Serra-Vinardell, J.; Roca-Ayats, N.; De-Ugarte, L.; Vilageliu, L.; Balcells, S.; Grinberg, D. Bone development and remodeling in metabolic disorders. J. Inherit. Metab. Dis. 2020, 43, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Chan, R.L.; Siu, W.S.; Shum, W.T.; Leung, P.C.; Zhang, L.; Cho, C.H. Deteriorating effect on bone metabolism and microstructure by passive cigarette smoking through dual actions on osteoblast and osteoclast. Calcif. Tissue Int. 2015, 96, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Franck, F.C.; Benatti, B.B.; Andia, D.C.; Cirano, F.R.; Casarin, R.C.; Correa, M.G.; Ribeiro, F.V. Impact of resveratrol on bone repair in rats exposed to cigarette smoke inhalation: Histomorphometric and bone-related gene expression analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 541–548. [Google Scholar] [CrossRef]
- Bai, P.; Sun, Y.; Jin, J.; Hou, J.; Li, R.; Zhang, Q.; Wang, Y. Disturbance of the OPG/RANK/RANKL pathway and systemic inflammation in COPD patients with emphysema and osteoporosis. Respir. Res. 2011, 12, 157. [Google Scholar] [CrossRef] [Green Version]
- Aliprantis, A.O.; Ueki, Y.; Sulyanto, R.; Park, A.; Sigrist, K.S.; Sharma, S.M.; Ostrowski, M.C.; Olsen, B.R.; Glimcher, L.H. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J. Clin. Investig. 2008, 118, 3775–3789. [Google Scholar] [CrossRef] [Green Version]
- Black, D.M.; Bauer, D.C.; Schwartz, A.V.; Cummings, S.R.; Rosen, C.J. Continuing bisphosphonate treatment for osteoporosis--for whom and for how long? N. Engl. J. Med. 2012, 366, 2051–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, Molecular Target, and Current Status on Drug Development. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wehner, C.; Lettner, S.; Moritz, A.; Andrukhov, O.; Rausch-Fan, X. Effect of bisphosphonate treatment of titanium surfaces on alkaline phosphatase activity in osteoblasts: A systematic review and meta-analysis. BMC Oral Health 2020, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Qi, M.; Wang, Y.; Feng, X.; Liu, H. Zoledronate and high glucose levels influence osteoclast differentiation and bone absorption via the AMPK pathway. BioChem. Biophys. Res. Commun. 2018, 505, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Niu, L.N.; Jiao, K.; Pei, D.D.; Pramanik, C.; Li, J.Y.; Messer, R.; Kumar, S.; Pashley, D.H.; Tay, F.R. Revival of nitrogen-containing bisphosphonate-induced inhibition of osteoclastogenesis and osteoclast function by water-soluble microfibrous borate glass. Acta BioMater. 2016, 31, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.; Cheng, C.C.; Chuang, P.Y.; Yang, T.Y. The effects of zoledronate on the survival and function of human osteoblast-like cells. BMC Musculoskelet. Disord. 2015, 16, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, G.I.; Qureshi, S.A.; Kenney, J.; Rubash, H.E.; Shanbhag, A.S. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 2004, 25, 4105–4115. [Google Scholar] [CrossRef]
- Bellido, T.; Plotkin, L.I. Novel actions of bisphosphonates in bone: Preservation of osteoblast and osteocyte viability. Bone 2011, 49, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yu, T.; Tang, H.; Ren, Z.; Li, Q.; Jia, J.; Chen, H.; Fu, J.; Ding, S.; Hao, Q.; et al. Ganoderma lucidum Immune Modulator Protein rLZ-8 Could Prevent and Reverse Bone Loss in Glucocorticoids-Induced Osteoporosis Rat Model. Front. Pharmacol. 2020, 11, 731. [Google Scholar] [CrossRef]
- Lenneras, M.; Palmquist, A.; Norlindh, B.; Emanuelsson, L.; Thomsen, P.; Omar, O. Oxidized Titanium Implants Enhance Osseointegration via Mechanisms Involving RANK/RANKL/OPG Regulation. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. 2), e486–e500. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.H.; Fitzsimmons, T.R.; Bartold, P.M. Effect of smoking on concentrations of receptor activator of nuclear factor kappa B ligand and osteoprotegerin in human gingival crevicular fluid. J. Clin. Periodontol. 2009, 36, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, B.; Farrugia, A.N.; To, L.B.; Findlay, D.M.; Green, J.; Lynch, K.; Zannettino, A.C. The nitrogen-containing bisphosphonate, zoledronic acid, influences RANKL expression in human osteoblast-like cells by activating TNF-alpha converting enzyme (TACE). J. Bone Miner. Res. 2004, 19, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Dobnig, H.; Hofbauer, L.C.; Viereck, V.; Obermayer-Pietsch, B.; Fahrleitner-Pammer, A. Changes in the RANK ligand/osteoprotegerin system are correlated to changes in bone mineral density in bisphosphonate-treated osteoporotic patients. Osteoporos. Int. 2006, 17, 693–703. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, G.S.; Jeong-Hwa, B. Inhibitory action of bisphosphonates on bone resorption does not involve the regulation of RANKL and OPG expression. Exp. Mol. Med. 2002, 34, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Verde, M.E.; Bermejo, D.; Gruppi, A.; Grenon, M. Effect of Bisphosphonates on the Levels of Rankl and Opg in Gingival Crevicular Fluid of Patients with Periodontal Disease and Post-menopausal Osteoporosis. Acta Odontol. Latinoam. 2015, 28, 215–221. [Google Scholar]
- Koch, F.P.; Merkel, C.; Ziebart, T.; Smeets, R.; Walter, C.; Al-Nawas, B. Influence of bisphosphonates on the osteoblast RANKL and OPG gene expression in vitro. Clin. Oral Investig. 2012, 16, 79–86. [Google Scholar] [CrossRef]
- D’Amelio, P.; Grimaldi, A.; Cristofaro, M.A.; Ravazzoli, M.; Molinatti, P.A.; Pescarmona, G.P.; Isaia, G.C. Alendronate reduces osteoclast precursors in osteoporosis. Osteoporos. Int. 2010, 21, 1741–1750. [Google Scholar] [CrossRef]
- Tsubaki, M.; Satou, T.; Itoh, T.; Imano, M.; Yanae, M.; Kato, C.; Takagoshi, R.; Komai, M.; Nishida, S. Bisphosphonate- and statin-induced enhancement of OPG expression and inhibition of CD9, M-CSF, and RANKL expressions via inhibition of the Ras/MEK/ERK pathway and activation of p38MAPK in mouse bone marrow stromal cell line ST2. Mol. Cell. Endocrinol. 2012, 361, 219–231. [Google Scholar] [CrossRef]
- Viereck, V.; Emons, G.; Lauck, V.; Frosch, K.H.; Blaschke, S.; Grundker, C.; Hofbauer, L.C. Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. BioChem. Biophys. Res. Commun. 2002, 291, 680–686. [Google Scholar] [CrossRef]
- Mulcahy, L.E.; Curtin, C.M.; McCoy, R.J.; O’Brien, F.J.; Taylor, D.; Lee, T.C.; Duffy, G.P. The effect of bisphosphonate treatment on the biochemical and cellular events during bone remodelling in response to microinjury stimulation. Eur. Cell Mater. 2015, 30, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, C.; Hitz, M.; Prieto-Alhambra, D.; Abrahamsen, B. Risks and Benefits of Bisphosphonate Therapies. J. Cell. BioChem. 2016, 117, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.C.; Karim, L.; Brooks, D.J.; Bouxsein, M.L.; Demay, M.B. Bisphosphonate Withdrawal: Effects on Bone Formation and Bone Resorption in Maturing Male Mice. J. Bone Miner. Res. 2017, 32, 814–820. [Google Scholar] [CrossRef] [Green Version]
- Wessel, J.H.; Dodson, T.B.; Zavras, A.I. Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: A case-control study. J. Oral Maxillofac. Surg. 2008, 66, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, W.; Haussling, V.; Aspera-Werz, R.H.; Springer, F.; Rinderknecht, H.; Braun, B.; Kuper, M.A.; Nussler, A.K.; Ehnert, S. Material-Dependent Formation and Degradation of Bone Matrix-Comparison of Two Cryogels. Bioengineering 2020, 7, 52. [Google Scholar] [CrossRef]
- Ehnert, S.; Linnemann, C.; Aspera-Werz, R.H.; Bykova, D.; Biermann, S.; Fecht, L.; De Zwart, P.M.; Nussler, A.K.; Stuby, F. Immune Cell Induced Migration of Osteoprogenitor Cells Is Mediated by TGF-beta Dependent Upregulation of NOX4 and Activation of Focal Adhesion Kinase. Int. J. Mol. Sci. 2018, 19, 2239. [Google Scholar] [CrossRef] [Green Version]
- Pasquier, J.; Thomas, B.; Hoarau-Vechot, J.; Odeh, T.; Robay, A.; Chidiac, O.; Dargham, S.R.; Turjoman, R.; Halama, A.; Fakhro, K.; et al. Circulating microparticles in acute diabetic Charcot foot exhibit a high content of inflammatory cytokines, and support monocyte-to-osteoclast cell induction. Sci. Rep. 2017, 7, 16450. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Tran, V.; Talbot, P. Comparison of toxicity of smoke from traditional and harm-reduction cigarettes using mouse embryonic stem cells as a novel model for preimplantation development. Hum. Reprod. 2009, 24, 386–397. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Han, W.; Giraldo, C.; De Li, Y.; Block, E.R. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. Am. J. Respir. Cell Mol. Biol. 1998, 19, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.X.; Gu, J.H.; Zhang, Y.R.; Tong, X.S.; Zhao, H.Y.; Yuan, Y.; Liu, X.Z.; Bian, J.C.; Liu, Z.P. Influence of osteoprotegerin on differentiation, activation, and apoptosis of Gaoyou duck embryo osteoclasts in vitro. Poult. Sci. 2013, 92, 1613–1620. [Google Scholar] [CrossRef]
- Bernhardt, A.; Koperski, K.; Schumacher, M.; Gelinsky, M. Relevance of osteoclast-specific enzyme activities in cell-based in vitro resorption assays. Eur. Cell Mater. 2017, 33, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, V.; Aspera-Werz, R.H.; Tendulkar, G.; Reumann, M.K.; Freude, T.; Breitkopf-Heinlein, K.; Dooley, S.; Pscherer, S.; Ochs, B.G.; Flesch, I.; et al. BMP9 a possible alternative drug for the recently withdrawn BMP7? New perspectives for (re-)implementation by personalized medicine. Arch. Toxicol. 2017, 91, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Giacomino, C.M.; Wealleans, J.A.; Kuhn, N.; Diogenes, A. Comparative Biocompatibility and Osteogenic Potential of Two Bioceramic Sealers. J. Endod. 2019, 45, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Zhao, J.; Pscherer, S.; Freude, T.; Dooley, S.; Kolk, A.; Stockle, U.; Nussler, A.K.; Hube, R. Transforming growth factor beta1 inhibits bone morphogenic protein (BMP)-2 and BMP-7 signaling via upregulation of Ski-related novel protein N (SnoN): Possible mechanism for the failure of BMP therapy? BMC Med. 2012, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Amin, N.; Boccardi, V.; Taghizadeh, M.; Jafarnejad, S. Probiotics and bone disorders: The role of RANKL/RANK/OPG pathway. Aging Clin. Exp. Res. 2020, 32, 363–371. [Google Scholar] [CrossRef]
- Yu, J.; Zanotti, S.; Schilling, L.; Canalis, E. Nuclear factor of activated T cells 2 is required for osteoclast differentiation and function in vitro but not in vivo. J. Cell. BioChem. 2018, 119, 9334–9345. [Google Scholar] [CrossRef]
- Feng, M.; Fang, F.; Fang, T.; Jiao, H.; You, S.; Wang, X.; Zhao, W. Sox13 promotes hepatocellular carcinoma metastasis by transcriptionally activating Twist1. Lab. Investig. 2020. [Google Scholar] [CrossRef]
- Ruoss, M.; Kieber, V.; Rebholz, S.; Linnemann, C.; Rinderknecht, H.; Haussling, V.; Hacker, M.; Olde Damink, L.H.H.; Ehnert, S.; Nussler, A.K. Cell-Type-Specific Quantification of a Scaffold-Based 3D Liver Co-Culture. Methods Protoc. 2019, 3, 1. [Google Scholar] [CrossRef] [Green Version]






| Gene | Accession Number | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Product Length (bp) | Annealing Temperature (°C) | Cycles |
|---|---|---|---|---|---|---|
| OPG | NM_002546.3 | CCGGAAACAGTGAATCAACTC | AGGTTAGCATGTCCAATGTG | 313 | 60 | 35 |
| RANKL | NM_033012.3 | TCCCAAGTTCTCATACCCTGA | CATCCAGGAAATACATAACAC | 245 | 56 | 35 |
| NFATC1 | NM_172390.2 | TGCAAGCCGAATTCTCTGGT | CTTTACGGCGACGTCGTTTC | 228 | 64 | 35 |
| β-Actin | NM_001101.3 | CGACAACGGTCCGGCATGT | GCACAGTGTGGGTGACCCCG | 461 | 64 | 30 |
| Antibody | Catalog No. | Company | Dilution |
|---|---|---|---|
| OPG | 500-P149 | Peprotech | 1:1000 |
| RANKL | 500-M46 | Peprotech | 1:1000 |
| TRAP 5b | Sc-376875 | Santa Cruz Biotech | 1:1000 |
| Goat anti-rabbit IgG-HRP | Sc-2004 | Santa Cruz Biotech | 1:10,000 |
| Goat anti-mouse IgM | Sc-2064 | Santa Cruz Biotech | 1:10,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Häussling, V.; Aspera-Werz, R.H.; Chen, T.; Braun, B.; Weng, W.; Histing, T.; Nussler, A.K. Bisphosphonates Reduce Smoking-Induced Osteoporotic-Like Alterations by Regulating RANKL/OPG in an Osteoblast and Osteoclast Co-Culture Model. Int. J. Mol. Sci. 2021, 22, 53. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010053
Zhu S, Häussling V, Aspera-Werz RH, Chen T, Braun B, Weng W, Histing T, Nussler AK. Bisphosphonates Reduce Smoking-Induced Osteoporotic-Like Alterations by Regulating RANKL/OPG in an Osteoblast and Osteoclast Co-Culture Model. International Journal of Molecular Sciences. 2021; 22(1):53. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010053
Chicago/Turabian StyleZhu, Sheng, Victor Häussling, Romina H. Aspera-Werz, Tao Chen, Bianca Braun, Weidong Weng, Tina Histing, and Andreas K. Nussler. 2021. "Bisphosphonates Reduce Smoking-Induced Osteoporotic-Like Alterations by Regulating RANKL/OPG in an Osteoblast and Osteoclast Co-Culture Model" International Journal of Molecular Sciences 22, no. 1: 53. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22010053