Synergic Effect of Selected Ingredients and Calcium Chloride on the Technological, Molecular and Microbial Usefulness of Eggshells and Their Impact on Sensory Properties in a Food Model System
Abstract
:1. Introduction
2. Results and Discussion
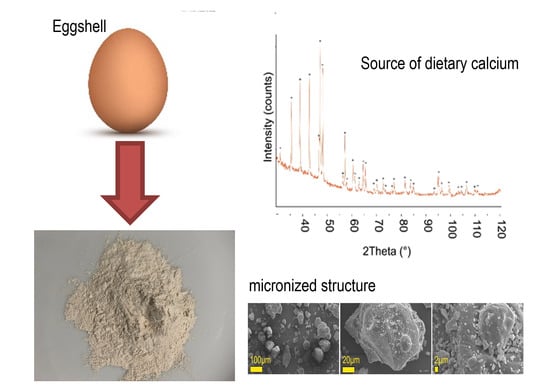
2.1. Analysis of Calcium Preparation Structure
2.1.1. Analysis of Morphology and Structure of Calcium Preparations
2.1.2. Determination of Hydrodynamic Diameter and Zeta Potential of Calcium Preparation Particles
2.2. Analysis of Physicochemical Properties of the Produced Preparations
2.3. Microbiological Analysis
2.4. Results of the Assessment of Sensory Quality of Food Products with the Addition of High-Protein Biofunctional Products
3. Materials and Methods
3.1. Material
3.1.1. Chemicals
3.1.2. Raw Materials
3.2. Sample Preparations
3.3. Structural Analyses
3.3.1. X-ray Diffraction
3.3.2. Scanning Electron Microscopy
3.3.3. Dynamic Light Scattering
3.3.4. Zeta Potential
3.4. Physicochemical Analyses
3.4.1. Determination of Active Acidity
3.4.2. Bulk Density
3.4.3. Ash Content
3.5. Microbiology
3.6. Implications of Chicken Eggshell Preparation for Food Products
3.7. Sensory Analysis of Products with Variants of Eggshell Preparations
3.8. Statistical Analysis
3.9. Safety Information
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goudarzi, F.; Mohammadalipour, A.; Khodadadi, I.; Karimi, S.; Mostoli, R.; Bahabadi, M.; Goodarzi, M.T. The Role of Calcium in Differentiation of Human Adipose-Derived Stem Cells to Adipocytes. Mol. Biotechnol. 2018, 60, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Beto, J.A. The Role of Calcium in Human Aging. Clin. Nutr. Res. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Hincke, M.T.; Leontovich, A.A.; Dronca, R.S.; Suman, V.J.; Ashdown, M.L.; Nevala, W.K.; Michael, A.; Robinson, A.; Kottschade, L.A.; Kaur, J.S.; et al. The eggshell: Structure, composition and mineralization. Front. Biosci. 2012, 17, 1266–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siemiradzka, W.; Dolinska, B.; Ryszka, F. New Sources of Calcium (Chicken Eggshells, Chelates)—Preparation of Raw Material and Tablets. Curr. Pharm. Biotechnol. 2018, 19, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Banaszewska, D.; Biesiada-Drzazga, B.; Marciniuk, M.; Hrnčár, C.; Arpášová, H.; Kaim-Mirowski, S. Comparison of the quality of cage and organic eggs available in retail and their content of selected macroelements. Acta Sci. Pol. Technol. Aliment. 2020, 19, 159–167. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.B.; Marie, P.; Nys, Y.; Hincke, M.T.; Gautron, J. Amorphous calcium carbonate controls avian eggshell mineralization: A new paradigm for understanding rapid eggshell calcification. J. Struct. Biol. 2015, 190, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Ketta, M.; Tůmová, E. Eggshell structure, measurements, and quality-affecting factors in laying hens: A review. Czech J. Anim. Sci. 2016, 61, 299–309. [Google Scholar] [CrossRef]
- Saeb, M.R.; Ghaffari, M.; Rastin, H.; Khonakdar, H.A.; Simon, F.; Najafi, F.; Goodarzi, V.; Vijayan, P.; Puglia, D.; Asl, F.H.; et al. Biowaste chicken eggshell powder as a potential cure modifier for epoxy/anhydride systems: Competitiveness with terpolymer-modified calcium carbonate at low loading levels. RSC Adv. 2017, 7, 2218–2230. [Google Scholar] [CrossRef] [Green Version]
- Dolińska, B.; Jelińska, M.; Szulc-Musioł, B.; Ryszka, F. Use of eggshells as a raw material for production of calcium preparations. Czech J. Food Sci. 2016, 34, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Orłowski, G.; Merta, D.; Pokorny, P.; Łukaszewicz, E.; Dobicki, W.; Kobielski, J.; Kowalczyk, A.; Rzońca, Z.; Krzywiński, A. Supporting dataset and methods for egg sizes, eggshell thicknesses and metal concentrations measured in the shells and contents of eggs of Capercaillies Tetrao urogallus. Data Br. 2019, 24, 103903. [Google Scholar] [CrossRef]
- Orłowski, G.; Hałupka, L.; Pokorny, P.; Klimczuk, E.; Sztwiertnia, H.; Dobicki, W.; Polechoński, R. The pattern of distribution and interaction of metals and calcium in eggshells and egg contents in relation to the embryonic development of eggs in a small passerine bird. J. Ornithol. 2017, 158, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health Effects and Sources of Prebiotic Dietary Fiber. Curr. Dev. Nutr. 2018, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef]
- Singh, P.; Prasad, S. Determination of ascorbic acid and its influence on the bioavailability of iron, zinc and calcium in Fijian food samples. Microchem. J. 2018, 139, 119–124. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell. Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef] [Green Version]
- Evenepoel, P.; Claes, K.; Meijers, B.; Laurent, M.; Bammens, B.; Naesens, M.; Sprangers, B.; Pottel, H.; Cavalier, E.; Kuypers, D. Poor Vitamin K Status Is Associated With Low Bone Mineral Density and Increased Fracture Risk in End-Stage Renal Disease. J. Bone Miner. Res. 2019, 34, 262–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhelazkova-Savova, M.; Gancheva, S.; Galunksa, B.; Gerova, D. Vitamin K: The new faces of an old friend—A role in bone and vascular health. Biomed. Rev. 2017, 28, 70–90. [Google Scholar] [CrossRef]
- Carrasco, F.; Basfi-fer, K.; Rojas, P.; Csendes, A.; Papapietro, K.; Codoceo, J.; Inostroza, J.; Krebs, N.F.; Westcott, J.L.; Miller, L.V.; et al. Calcium absorption may be affected after either sleeve gastrectomy or Roux-en-Y gastric bypass in premenopausal women: A 2-y prospective study. Am. J. Clin. Nutr. 2018, 108, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Brun, L.R.; Lupo, M.; Delorenzi, D.A.; Di Loreto, V.E.; Rigalli, A. Chicken eggshell as suitable calcium source at home. Int. J. Food Sci. Nutr. 2013, 64, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Barman, A.K.; Roy, P.K.; Singh, B.K. Chicken eggshell powder as dietary calcium source in chocolate cakes. Pharma Innov. J. 2017, 6, 01–04. [Google Scholar]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szymandera-Buszka, K.; Rezler, R.; Jarzębski, M.; Szczepaniak, O.; Marciniak, G.; Jędrusek-Golińska, A.; Kobus-Moryson, M. Effect of fortification with calcium from eggshells on bioavailability, quality, and rheological characteristics of traditional Polish bread spread. J. Dairy Sci. 2020. [Google Scholar] [CrossRef]
- Penfield, M.P.; Campbell, A.M. Evaluating food by sensory methods. In Experimental Food Science; Elsevier: Amsterdam, The Netherlands, 1990; pp. 51–77. [Google Scholar]
- Mohammadi, X.; Deng, Y.; Matinfar, G.; Singh, A.; Mandal, R.; Pratap-Singh, A. Impact of Three Different Dehydration Methods on Nutritional Values and Sensory Quality of Dried Broccoli, Oranges, and Carrots. Foods 2020, 9, 1464. [Google Scholar] [CrossRef]
- Jarzębski, M.; Bellich, B.; Białopiotrowicz, T.; Śliwa, T.; Kościński, J.; Cesàro, A. Particle tracking analysis in food and hydrocolloids investigations. Food Hydrocoll. 2017, 68, 90–101. [Google Scholar] [CrossRef]
- Jarzębski, M.; Fathordoobady, F.; Guo, Y.; Xu, M.; Singh, A.; Kitts, D.D.; Kowalczewski, P.Ł.; Jeżowski, P.; Pratap Singh, A. Pea Protein for Hempseed Oil Nanoemulsion Stabilization. Molecules 2019, 24, 4288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarzębski, M.; Siejak, P.; Smułek, W.; Fathordoobady, F.; Guo, Y.; Pawlicz, J.; Trzeciak, T.; Kowalczewski, P.Ł.; Kitts, D.D.; Singh, A.; et al. Plant Extracts Containing Saponins Affects the Stability and Biological Activity of Hempseed Oil Emulsion System. Molecules 2020, 25, 2696. [Google Scholar] [CrossRef]
- Jarzębski, M.; Smułek, W.; Baranowska, H.M.; Masewicz, Ł.; Kobus-Cisowska, J.; Ligaj, M.; Kaczorek, E. Characterization of St. John’s wort (Hypericum perforatum L.) and the impact of filtration process on bioactive extracts incorporated into carbohydrate-based hydrogels. Food Hydrocoll. 2020, 104, 105748. [Google Scholar] [CrossRef]
- Jarzębski, M.; Smułek, W.; Siejak, P.; Rezler, R.; Pawlicz, J.; Trzeciak, T.; Jarzębska, M.; Majchrzak, O.; Kaczorek, E.; Kazemian, P.; et al. Aesculus hippocastanum L. as a Stabilizer in Hemp Seed Oil Nanoemulsions for Potential Biomedical and Food Applications. Int. J. Mol. Sci. 2021, 22, 887. [Google Scholar] [CrossRef]
- Szulc, K.; Lenart, A. Właściwości kohezyjne wybranych proszków spożywczych [Cohesion properties of selected food powders]. Inżynieria Rol. 2009, 13, 169–175. [Google Scholar]
- Szeleszczuk, Ł.; Pisklak, D.M.; Kuras, M.; Wawer, I. In Vitro Dissolution of Calcium Carbonate from the Chicken Eggshell: A Study of Calcium Bioavailability. Int. J. Food Prop. 2015, 18, 2791–2799. [Google Scholar] [CrossRef]
- Hassan, N.M. Chicken Eggshell Powder as Dietary Calcium Source in Biscuits. World J. Dairy Food Sci. 2015, 10, 199–206. [Google Scholar] [CrossRef]
- Feng, Y.; Tapia, M.A.; Okada, K.; Castaneda Lazo, N.B.; Chapman-Novakofski, K.; Phillips, C.; Lee, S.-Y. Consumer Acceptance Comparison Between Seasoned and Unseasoned Vegetables. J. Food Sci. 2018, 83, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Szymandera-Buszka, K.; Jędrusek-Goliśnka, A.; Waszkowiak, K.; Hęś, M. Sensory sensitivity to sour and bitter taste among people with Crohn’s disease and folic acid supplementation. J. Sens. Sci. 2020, 35, e12550. [Google Scholar] [CrossRef]
- Drewnowski, A. The Science and Complexity of Bitter Taste. Nutr. Rev. 2001, 59, 163–169. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Krawczyk, J.; Puchała, M.; Józefiak, D. Dietary factors improving eggshell quality: An updated review with special emphasis on microelements and feed additives. World Poultry Sci. J. 2015, 71, 83–94. [Google Scholar] [CrossRef]
- Saeed, M.; Yasmin, I.; Pasha, I.; Randhawa, M.A.; Khan, M.I.; Shabbir, M.A.; Khan, W.A. Potential application of inulin in food industry; a review. Pak. J. Food Sci. 2015, 25, 110–116. [Google Scholar]
- Berne, B.J.; Pecora, R. Dynamical Light Scattering with Reference to Physics, Chemistry and Biology; Wiley/Interscience: New York, NY, USA, 1976. [Google Scholar]
- PN-EN ISO 2171:2010 Cereals, Pulses and by-Products—Determination of Ash Yield by Incineration; Polish Standardising Commitee: Warsaw, Poland, 2010.
- PN-A-75052-05:1990 Fruit, Vegetable and Vegetable-Meat Preparations—Methods of Microbiological Tests—Determination of the Presence and Number of Mesophilic and Psychrophilic Aerobic Microorganisms; Polish Standardising Commitee: Warsaw, Poland, 1990.
- PN-A-75052-07:1990 Fruit, Vegetable and Vegetable-Meat Preparations—Methods of Microbiological Tests—Determination of the Number of Lactic Acid Bacteria; Polish Standardising Commitee: Warsaw, Poland, 1990.
- PN-ISO 4832:2007 Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique; Polish Standardising Commitee: Warsaw, Poland, 2007.
- PN-EN ISO 6579-1:2017-04 Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part. 1: Detection of Salmonella spp.; Polish Standardising Commitee: Warsaw, Poland, 2017.
- PN-EN ISO 21528-2:2017-08 Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part. 2: Colony-Count Technique; Polish Standardising Commitee: Warsaw, Poland, 2017.




| MODEL | d15 (nm) | PDI (–) | d90 (nm) | PDI (–) | d175 (nm) | PDI (–) | ζ ± SD (mV) | EM (μm·cm·s−1·V−1) |
|---|---|---|---|---|---|---|---|---|
| EGR | 917 | 32 | 370 | 28 | 372 | 24 | 21.0 ± 0.4 | –16,352 |
| EHSI | 1053 | 31 | 698 | 37 | 1143 | 22 | 18.5 ± 0.8 | –14,419 |
| ELI | 602 | 37 | 327 | 18 | 346 | 27 | 23.6 ± 0.4 | –18,400 |
| ELA | 875 | 26 | 425 | 33 | 358 | 29 | 20.7 ± 0.5 | –16,165 |
| ED | 785 | 18 | 451 | 27 | 677 | 27 | 20.5 ± 0.4 | –15,971 |
| ECM | 2197 | 39 | 1049 | 27 | 1263 | 24 | 18.1 ± 0.4 | –14,119 |
| EK | 2064 | 35 | 1983 | 33 | 2174 | 16 | 19.5 ± 0.3 | –15,178 |
| EC | 934 | 22 | 501 | 27 | 752 | 26 | 19.1 ± 0.5 | –14,897 |
| MODEL | pH | Bulk Density (g/cm3) | Ash (% Dry Matter) |
|---|---|---|---|
| EGR | 9.36 ab ± 0.01 | 0.86 b ± 0.02 | 37.35 e ± 0.37 |
| EHSI | 9.50 a ± 0.14 | 0.90 b ± 0.01 | 34.20 f ± 0.12 |
| ELI | 7.58 f ± 0.01 | 0.58 e ± 0.01 | 51.55 a ± 0.81 |
| ELA | 9.04 d ± 0.01 | 0.70 d ± 0.01 | 34.30 f ± 0.17 |
| ED | 9.39 ab ± 0.01 | 1.07 a ± 0.03 | 51.80 a ± 0.40 |
| ECM | 8.75 e ± 0.07 | 0.86 b ± 0.05 | 71.90 b ± 0.75 |
| EK | 9.28 abd ± 0.02 | 0.73 cd ± 0.01 | 54.10 c ± 0.08 |
| EC | 8.34 bd ± 0.07 | 0.43 f ± 0.00 | 55.40 d ± 0.39 |
| Variable | Factor–Variable Correlations (Factor Loadings), Based on Correlations | ||||||
|---|---|---|---|---|---|---|---|
| EC | EGR | EHSI | ELI | ELA | ED | ECM | |
| Meatballs | |||||||
| meat taste | 7.5 b | 5.0 a | 5.5 a | 6.0 a | 6.2 a | 6.0 a | 6.1 a |
| salty taste | 3.0 a | 3.1 a | 3.4 a | 3.0 a | 3.3 a | 3.3 a | 3.2 a |
| bitter taste | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 1.0 b |
| fatty taste | 3.1 a | 3.2 a | 3.1 a | 3.1 a | 3.2 a | 3.1 a | 3.0 a |
| powdered taste | 0.0 a | 0.3 a | 0.2 a | 0.0 a | 0.0 a | 0.0 a | 1.5 b |
| metallic taste | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a |
| red color | 1.2 a | 1.3 a | 1.2 a | 1.2 a | 1.5 a | 1.2 a | 1.3 a |
| gray color | 1.6 a | 1.6 a | 1.3 a | 1.8 a | 1.6 a | 1.5 a | 1.3 a |
| brown color | 6.5 a | 6.4 a | 6.4 a | 6.6 a | 6.4 a | 6.3 a | 6.4 a |
| uniformity of color | 8.5 a | 8.5 a | 8.5 a | 8.5 a | 8.5 a | 8.5 a | 8.5 a |
| taste desirability | 9.0 a | 8.8 a | 9.2 a | 9.0 a | 9.1 a | 9.0 a | 9.0 a |
| color desirability | 9.0 a | 9.0 a | 9.0 a | 8.9 a | 9.0 a | 9.1 a | 9.0 a |
| general desirability | 9.0 a | 9.0 a | 9.0 a | 8.9 a | 9.0 a | 9.0 a | 9.2 a |
| Jelly fruit dessert | |||||||
| sour taste | 3.7 b | 2.0 a | 2.0 a | 2.0 a | 2.2 a | 1.5 a | 1.6 a |
| sweet taste | 4.2 c,b | 3.1 a | 3.6 b,a | 3.0 a | 4.5 c,b | 3.3 a | 3.2 a |
| bitter taste | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.4 b | 0.7 a |
| fruit taste | 5.2 a | 4.4 a | 4.3 a | 4.3 a | 4.9 a | 4.3 a | 4.2 a |
| mango taste | 4.2 a | 3.5 a | 3.4 a | 3.5 a | 4.3 a | 3.6 a | 3.6 a |
| powdered taste | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.7 a |
| metallic taste | 0.0 a | 0.0 a | 0.0 a | 0.5 b | 0.0 a | 0.5 b | 0.5 b |
| yellow color | 4.5 a | 4.6 a | 4.5 a | 4.7 a | 4.7 a | 4.5 a | 4.5 a |
| brown color | 0.5 a | 0.5 a | 0.6 a | 0.7 a | 0.5 a | 0.4 a | 0.5 a |
| clarity of color | 2.8 a | 3.0 a | 2.8 a | 2.9 a | 3.1 a | 3.2 a | 2.8 a |
| uniformity of color | 9.0 a | 9,2 a | 9.0 a | 9.1 a | 9.2 a | 9.2 a | 9.3 a |
| taste desirability | 9.0 a | 9.0 a | 9.2 a | 9.1 a | 9.1 a | 9.0 a | 8.9 a |
| color desirability | 9.0 a | 9.0 a | 9.0 a | 9.0 a | 9.0 a | 9.1 a | 9.0 a |
| general desirability | 8.0 a | 8.2 a | 8.2 a | 8.0 a | 8.0 a | 8.3 a | 8.4 a |
| Apple juice with mango | |||||||
| sour taste | 4.3 b | 3.8 b,a | 4.4 b | 3.5 a | 3.3 a | 3.5 a | 3.4 a |
| sweet taste | 4.2 c,b | 3.6 b,a | 4.1 b | 3.0 a | 4.5 c | 3.1 a | 3.2 a |
| bitter taste | 0.0 a | 0.0 a | 0.0 a | 0.2 a | 0.0 a | 0.4 a | 0.6 a |
| fruit taste | 7.1 a | 6.0 a | 6.4 a | 6.6 a | 6.5 a | 6.6 a | 6.2 a |
| mango taste | 5.4 a | 5.0 a | 5.0 a | 4.8 a | 4.9 a | 5.1 a | 4.8 a |
| powdered taste | 0.0 a | 0.0 a | 0.2 a | 0.0 a | 0.0 a | 0.0 a | 0.4 a |
| metallic taste | 0.0 a | 0.0 a | 0.0 a | 0.5 b | 0.0 a | 0.5 b | 0.5 b |
| yellow color | 5.6 a | 5.5 a | 5.6 a | 5.4 a | 5.6 a | 5.6 a | 5.6 a |
| brown color | 0.5 a | 0.5 a | 0.6 a | 0.7 a | 0.5 a | 0.4 a | 0.5 a |
| clarity of color | 2.6 a | 2.4 a | 2.4 a | 2.5 a | 2.7 a | 2.8 a | 2.7 a |
| uniformity of color | 9.0 a | 9.2 a | 9.0 a | 9.1 a | 9.0 a | 9.2 a | 9.0 a |
| taste desirability | 9.0 a | 9.0 a | 9.0 a | 9.1 a | 9.1 a | 9.0 a | 9.2 a |
| color desirability | 9.0 a | 9.0 a | 9.0 a | 9.0 a | 9.0 a | 9.1 a | 9.0 a |
| general desirability | 8.5 a | 8.3 a | 8.2 a | 8.2 a | 8.4 a | 8.3 a | 8.6 a |
| EGR | EHSI | ELI | ELA | ED | ECM | EK | EC | |
|---|---|---|---|---|---|---|---|---|
| Ingredient | Content in preparation (%) | |||||||
| Eggshell | 35 | 35 | 35 | 35 | 87.5 | 50 | 96 | 100 |
| Inulin GR | 65 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Inulin HSI | 0 | 65 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lysine | 0 | 0 | 65 | 0 | 0 | 0 | 0 | 0 |
| Lactose | 0 | 0 | 0 | 65 | 0 | 0 | 0 | 0 |
| Vitamin D3 | 0 | 0 | 0 | 0 | 12.5 | 0 | 0 | 0 |
| Magnesium chloride | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 0 |
| Vitamin K | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymandera-Buszka, K.; Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Rezler, R.; Szczepaniak, O.; Marciniak, G.; Piechocka, J.; Jarzębski, M. Synergic Effect of Selected Ingredients and Calcium Chloride on the Technological, Molecular and Microbial Usefulness of Eggshells and Their Impact on Sensory Properties in a Food Model System. Int. J. Mol. Sci. 2021, 22, 2029. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22042029
Szymandera-Buszka K, Kobus-Cisowska J, Szymanowska-Powałowska D, Rezler R, Szczepaniak O, Marciniak G, Piechocka J, Jarzębski M. Synergic Effect of Selected Ingredients and Calcium Chloride on the Technological, Molecular and Microbial Usefulness of Eggshells and Their Impact on Sensory Properties in a Food Model System. International Journal of Molecular Sciences. 2021; 22(4):2029. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22042029
Chicago/Turabian StyleSzymandera-Buszka, Krystyna, Joanna Kobus-Cisowska, Daria Szymanowska-Powałowska, Ryszard Rezler, Oskar Szczepaniak, Grzegorz Marciniak, Justyna Piechocka, and Maciej Jarzębski. 2021. "Synergic Effect of Selected Ingredients and Calcium Chloride on the Technological, Molecular and Microbial Usefulness of Eggshells and Their Impact on Sensory Properties in a Food Model System" International Journal of Molecular Sciences 22, no. 4: 2029. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22042029