Elucidating Carfilzomib’s Induced Cardiotoxicity in an In Vivo Model of Aging: Prophylactic Potential of Metformin
Abstract
:1. Introduction
2. Results
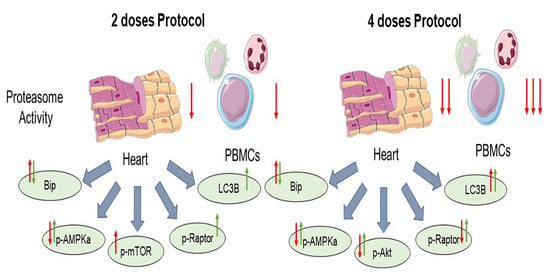
2.1. Carfilzomib Administration of Two and Four Doses Decreased Proteasome Activity, % Fractional Shortening and Increased Myocardial Oxidative Stress
2.2. Carfilzomib Administration (2 Doses Protocol) Reduced AMPKα Phosphorylation and Increased Bip Expression, without Increasing PP2A Activity
2.3. Carfilzomib Administration (Four-Dose Protocol) Increased PP2A Activity, Reduced AMPKα Phosphorylation and Increased Bip and LC3B-Depedendent Autophagy
2.4. Metformin Did Not Interfere with Carfilzomib’s Proteasome Inhibitory Activity, Restored MDA Levels in the Myocardium, and Maintained LV Function in the Two Doses Protocol
2.5. Metformin Mitigated Early Cfz Cardiotoxicity in the Two-Dose Regimen, through Induction of AMPKα Signaling and Synergistic Increase in LC3B
2.6. Metformin Did Not Interfere with Carfilzomib’s Proteasome Inhibitory Potential and Maintained LV Function in the Four-Dose Protocol, Independently of Myocardial Oxidative Stress
2.7. Coadministration of Cfz with Metformin Reduced Myocardial PP2A Activity, Partially Restored Bip Expression and Increased AMPKα Phosphorylation Inducing LC3B-Dependent Autophagy in the Aged Myocardium
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. PBMCs Isolation
4.3. Measurement of Proteasome Peptidase Activity
4.4. Echocardiography
4.5. Western Blot Analysis
4.6. Myocardial MDA Content
4.7. PP2A Activity Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orlowski, R.Z.; Moreau, P.; Niesvizky, R.; Ludwig, H.; Oriol, A.; Chng, W.J.; Goldschmidt, H.; Yang, Z.; Kimball, A.S.; Dimopoulos, M. Carfilzomib-Dexamethasone Versus Bortezomib-Dexamethasone in Relapsed or Refractory Multiple Myeloma: Updated Overall Survival, Safety, and Subgroups. Clin. Lymphoma Myeloma Leuk. 2019, 19, 522–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimopoulos, M.A.; Goldschmidt, H.; Niesvizky, R.; Joshua, D.; Chng, W.J.; Oriol, A.; Orlowski, R.Z.; Ludwig, H.; Facon, T.; Hajek, R.; et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): An interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1327–1337. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Quach, H.; Mateos, M.V.; Landgren, O.; Leleu, X.; Siegel, D.; Weisel, K.; Yang, H.; Klippel, Z.; Zahlten-Kumeli, A.; et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomised, multicentre, open-label, phase 3 study. Lancet 2020, 396, 186–197. [Google Scholar] [CrossRef]
- Ziogas, D.C.; Terpos, E.; Kastritis, E.; Dimopoulos, M.A. An overview of the role of carfilzomib in the treatment of multiple myeloma. Expert Opin. Pharmacother. 2017, 18, 1883–1897. [Google Scholar] [CrossRef]
- Bringhen, S.; Milan, A.; D’Agostino, M.; Ferri, C.; Wasch, R.; Gay, F.; Larocca, A.; Offidani, M.; Zweegman, S.; Terpos, E.; et al. Prevention, monitoring and treatment of cardiovascular adverse events in myeloma patients receiving carfilzomib A consensus paper by the European Myeloma Network and the Italian Society of Arterial Hypertension. J. Intern. Med. 2019, 286, 63–74. [Google Scholar] [CrossRef]
- Efentakis, P.; Kremastiotis, G.; Varela, A.; Nikolaou, P.E.; Papanagnou, E.D.; Davos, C.H.; Tsoumani, M.; Agrogiannis, G.; Konstantinidou, A.; Kastritis, E.; et al. Molecular mechanisms of carfilzomib-induced cardiotoxicity in mice and the emerging cardioprotective role of metformin. Blood 2019, 133, 710–723. [Google Scholar] [CrossRef] [Green Version]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Zweegman, S.; Palumbo, A.; Bringhen, S.; Sonneveld, P. Age and aging in blood disorders: Multiple myeloma. Haematologica 2014, 99, 1133–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anagnostopoulos, A.; Gika, D.; Symeonidis, A.; Zervas, K.; Pouli, A.; Repoussis, P.; Grigoraki, V.; Anagnostopoulos, N.; Economopoulos, T.; Maniatis, A.; et al. Multiple myeloma in elderly patients: Prognostic factors and outcome. Eur. J. Haematol. 2005, 75, 370–375. [Google Scholar] [CrossRef]
- Diamond, E.; Lahoud, O.B.; Landau, H. Managing multiple myeloma in elderly patients. Leuk. Lymphoma 2018, 59, 1300–1311. [Google Scholar] [CrossRef]
- Crimmins, E.M.; Shim, H.; Zhang, Y.S.; Kim, J.K. Differences between Men and Women in Mortality and the Health Dimensions of the Morbidity Process. Clin. Chem. 2019, 65, 135–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seals, D.R.; Brunt, V.E.; Rossman, M.J. Keynote lecture: Strategies for optimal cardiovascular aging. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H183–H188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triposkiadis, F.; Xanthopoulos, A.; Butler, J. Cardiovascular Aging and Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 804–813. [Google Scholar] [CrossRef]
- Daiber, A.; Hahad, O.; Andreadou, I.; Steven, S.; Daub, S.; Munzel, T. Redox-related biomarkers in human cardiovascular disease—Classical footprints and beyond. Redox Biol. 2021, 42, 101875. [Google Scholar] [CrossRef]
- Oslowski, C.M.; Urano, F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011, 490, 71–92. [Google Scholar]
- Reddy, P.; Shenoy, C.; Blaes, A.H. Cardio-oncology in the older adult. J. Geriatr. Oncol. 2017, 8, 308–314. [Google Scholar] [CrossRef]
- Olivetti, G.; Melissari, M.; Capasso, J.M.; Anversa, P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ. Res. 1991, 68, 1560–1568. [Google Scholar] [CrossRef] [Green Version]
- Vilchez, D.; Saez, I.; Dillin, A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat. Commun. 2014, 5, 5659. [Google Scholar] [CrossRef]
- Gergs, U.; Trapp, T.; Bushnaq, H.; Simm, A.; Silber, R.E.; Neumann, J. Age-Dependent Protein Expression of Serine/Threonine Phosphatases and Their Inhibitors in the Human Cardiac Atrium. Adv. Med. 2019, 2019, 2675972. [Google Scholar] [CrossRef]
- Kastle, M.; Grune, T. Protein oxidative modification in the aging organism and the role of the ubiquitin proteasomal system. Curr. Pharm. Des. 2011, 17, 4007–4022. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Jakubowiak, A.J.; McCarthy, P.L.; Orlowski, R.Z.; Attal, M.; Blade, J.; Goldschmidt, H.; Weisel, K.C.; Ramasamy, K.; Zweegman, S.; et al. Developments in continuous therapy and maintenance treatment approaches for patients with newly diagnosed multiple myeloma. Blood Cancer J. 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glembotski, C.C. The role of the unfolded protein response in the heart. J. Mol. Cell. Cardiol. 2008, 44, 453–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Wang, X.; Gillette, T.G.; Deng, Y.; Wang, Z.V. Unfolded Protein Response as a Therapeutic Target in Cardiovascular Disease. Curr. Top. Med. Chem. 2019, 19, 1902–1917. [Google Scholar] [CrossRef]
- Estebanez, B.; de Paz, J.A.; Cuevas, M.J.; Gonzalez-Gallego, J. Endoplasmic Reticulum Unfolded Protein Response, Aging and Exercise: An Update. Front. Physiol. 2018, 9, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Ni, M.; Lee, B.; Barron, E.; Hinton, D.R.; Lee, A.S. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008, 15, 1460–1471. [Google Scholar] [CrossRef]
- Efentakis, P.; Doerschmann, H.; Witzler, C.; Siemer, S.; Nikolaou, P.E.; Kastritis, E.; Stauber, R.; Dimopoulos, M.A.; Wenzel, P.; Andreadou, I.; et al. Investigating the Vascular Toxicity Outcomes of the Irreversible Proteasome Inhibitor Carfilzomib. Int. J. Mol. Sci. 2020, 21, 5185. [Google Scholar] [CrossRef]
- Kitakaze, M.; Tsukamoto, O. What Is the Role of ER Stress in the Heart? Introduction and Series Overview. Circ. Res. 2010, 107, 15–18. [Google Scholar] [CrossRef]
- Hwang, S.L.; Jeong, Y.T.; Li, X.; Kim, Y.D.; Lu, Y.; Chang, Y.C.; Lee, I.K.; Chang, H.W. Inhibitory cross-talk between the AMPK and ERK pathways mediates endoplasmic reticulum stress-induced insulin resistance in skeletal muscle. Br. J. Pharmacol. 2013, 169, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Rashid, H.O.; Yadav, R.K.; Kim, H.R.; Chae, H.J. ER stress: Autophagy induction, inhibition and selection. Autophagy 2015, 11, 1956–1977. [Google Scholar] [CrossRef] [PubMed]
- Efentakis, P.; Varela, A.; Chavdoula, E.; Sigala, F.; Sanoudou, D.; Tenta, R.; Gioti, K.; Kostomitsopoulos, N.; Papapetropoulos, A.; Tasouli, A.; et al. Levosimendan prevents doxorubicin-induced cardiotoxicity in time- and dose dependent manner: Implications for inotropy. Cardiovasc. Res. 2019, 116, 576–591. [Google Scholar] [CrossRef]
- Chari, A.; Hajje, D. Case series discussion of cardiac and vascular events following carfilzomib treatment: Possible mechanism, screening, and monitoring. BMC Cancer 2014, 14, 915. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, J.; Saguner, A.M.; Versari, D.; Peterson, T.E.; Chade, A.; Olson, M.; Lerman, L.O.; Lerman, A. Chronic proteasome inhibition contributes to coronary atherosclerosis. Circ. Res. 2007, 101, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, J.; Wohlert, C.; Saguner, A.M.; Flores, A.; Nesbitt, L.L.; Chade, A.; Lerman, L.O.; Lerman, A. Primary proteasome inhibition results in cardiac dysfunction. Eur. J. Heart Fail. 2013, 15, 614–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.F.; Zhang, J.Y.; Li, L.; Zhao, X.Y.; Tao, H.L.; Zhang, L. Metformin improves cardiac function in rats via activation of AMP-activated protein kinase. Clin. Exp. Pharmacol. Physiol. 2011, 38, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.B.; Sundaresan, N.R.; Gupta, M.P. Regulation of Akt signaling by sirtuins: Its implication in cardiac hypertrophy and aging. Circ. Res. 2014, 114, 368–378. [Google Scholar] [CrossRef] [Green Version]
- Salt, I.P.; Hardie, D.G. AMP-Activated Protein Kinase: An Ubiquitous Signaling Pathway With Key Roles in the Cardiovascular System. Circ. Res. 2017, 120, 1825–1841. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zou, M.H. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4987. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell. Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Tai, H.; Wang, X.; Wang, Z.; Zhou, J.; Wei, X.; Ding, Y.; Gong, H.; Mo, C.; Zhang, J.; et al. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+) elevation. Aging Cell 2016, 15, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Docrat, T.F.; Nagiah, S.; Naicker, N.; Baijnath, S.; Singh, S.; Chuturgoon, A.A. The protective effect of metformin on mitochondrial dysfunction and endoplasmic reticulum stress in diabetic mice brain. Eur. J. Pharmacol. 2020, 875, 173059. [Google Scholar] [CrossRef]
- Song, Y.M.; Lee, W.K.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. Metformin Restores Parkin-Mediated Mitophagy, Suppressed by Cytosolic p53. Int. J. Mol. Sci. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theriault, J.R.; Palmer, H.J.; Pittman, D.D. Inhibition of the Unfolded Protein Response by metformin in renal proximal tubular epithelial cells. Biochem. Biophys. Res. Commun. 2011, 409, 500–505. [Google Scholar] [CrossRef]
- Fotiou, D.; Roussou, M.; Gakiopoulou, C.; Psimenou, E.; Gavriatopoulou, M.; Migkou, M.; Kanellias, N.; Dialoupi, I.; Eleutherakis-Papaiakovou, E.; Giannouli, S.; et al. Carfilzomib-associated renal toxicity is common and unpredictable: A comprehensive analysis of 114 multiple myeloma patients. Blood Cancer J. 2020, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ri, M.; Chou, T.; Sugiura, I.; Takezako, N.; Sunami, K.; Ishida, T.; Izumi, T.; Ozaki, S.; Shumiya, Y.; et al. Carfilzomib, lenalidomide and dexamethasone in patients with heavily pretreated multiple myeloma: A phase 1 study in Japan. Cancer Sci. 2017, 108, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Bringhen, S.; Petrucci, M.T.; Larocca, A.; Conticello, C.; Rossi, D.; Magarotto, V.; Musto, P.; Boccadifuoco, L.; Offidani, M.; Omede, P.; et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: A multicenter, phase 2 study. Blood 2014, 124, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackert-Bicknell, C.L.; Anderson, L.C.; Sheehan, S.; Hill, W.G.; Chang, B.; Churchill, G.A.; Chesler, E.J.; Korstanje, R.; Peters, L.L. Aging Research Using Mouse Models. Curr. Protoc. Mouse Biol. 2015, 5, 95–133. [Google Scholar] [CrossRef] [Green Version]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012, 20, 256–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaou, P.E.; Efentakis, P.; Abu Qourah, F.; Femmino, S.; Makridakis, M.; Kanaki, Z.; Varela, A.; Tsoumani, M.; Davos, C.H.; Dimitriou, C.A.; et al. Chronic Empagliflozin Treatment Reduces Myocardial Infarct Size in Nondiabetic Mice Through STAT-3-Mediated Protection on Microvascular Endothelial Cells and Reduction of Oxidative Stress. Antioxid. Redox Signal. 2021, 34, 551–571. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efentakis, P.; Psarakou, G.; Varela, A.; Papanagnou, E.D.; Chatzistefanou, M.; Nikolaou, P.-E.; Davos, C.H.; Gavriatopoulou, M.; Trougakos, I.P.; Dimopoulos, M.A.; et al. Elucidating Carfilzomib’s Induced Cardiotoxicity in an In Vivo Model of Aging: Prophylactic Potential of Metformin. Int. J. Mol. Sci. 2021, 22, 10956. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222010956
Efentakis P, Psarakou G, Varela A, Papanagnou ED, Chatzistefanou M, Nikolaou P-E, Davos CH, Gavriatopoulou M, Trougakos IP, Dimopoulos MA, et al. Elucidating Carfilzomib’s Induced Cardiotoxicity in an In Vivo Model of Aging: Prophylactic Potential of Metformin. International Journal of Molecular Sciences. 2021; 22(20):10956. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222010956
Chicago/Turabian StyleEfentakis, Panagiotis, Garyfalia Psarakou, Aimilia Varela, Eleni Dimitra Papanagnou, Michail Chatzistefanou, Panagiota-Efstathia Nikolaou, Costantinos H. Davos, Maria Gavriatopoulou, Ioannis P. Trougakos, Meletios Athanasios Dimopoulos, and et al. 2021. "Elucidating Carfilzomib’s Induced Cardiotoxicity in an In Vivo Model of Aging: Prophylactic Potential of Metformin" International Journal of Molecular Sciences 22, no. 20: 10956. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222010956