Building up and Characterization of Calcined Marl-Based Geopolymeric Cement
Abstract
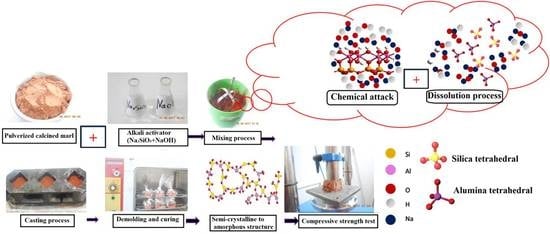
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
3. Results and Discussion
3.1. Chemical Composition of Aluminosilicate Source
3.2. Mineral Composition of Aluminosilicate Source
3.3. Mineral Transformation on Calcination
3.4. Compressive Strength Performance
3.4.1. Effect of NaOH Concentration
3.4.2. Effect of Na2SiO3/NaOH (Sodium Silicate/Sodium Hydroxide (SS/SH)) Mass Ratio
3.4.3. Effect of Solid/Liquid (S/L) Mass Ratio
3.4.4. Effect of Curing Temperatures
3.4.5. Effect of Calcination Temperatures
3.4.6. Effect of Curing Time
3.4.7. Effect of Aging Time
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 35, 429–441. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Sirivivatnanon, V. Workability and strength of coarse high calcium fly ash geopolymer. Cem. Concr. Compos. 2007, 29, 224–229. [Google Scholar] [CrossRef]
- Mobili, A.; Belli, A.; Giosuè, C.; Bellezze, T.; Tittarelli, F. Metakaolin and fly ash alkali-activated mortars compared with cementitious mortars at the same strength class. Cem. Concr. Res. 2016, 88, 198–210. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palamo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Rovanik, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 27, 1176–1183. [Google Scholar] [CrossRef]
- Favier, A.; Habert, G.; Roussel, N.; de Laceillerie, J.B. A multinuclear static NMR study of geopolymerisation. Cem. Concr. Res. 2015, 75, 104–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, W.; Chen, Q.; Chen, L. Synthesis and heavy metal immobilization behaviors of slag based geopolymer. J. Hazard. Mater. 2007, 143, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, F.; Roviello, G.; Ricciotti, L.; Ferrándiz-Mass, V.; Messina, F.; Ferone, C.; Tarallo, O.; Cioffi, R.; Cheeseman, C.R. Mechanical and thermal properties of lightweight geopolymer composites. Cem. Concr. Compos. 2018, 86, 266–272. [Google Scholar] [CrossRef]
- Monteiro, P.J.; Mehta, M.P. Concrete: Structure, Properties and Materials, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1993; pp. 21–23. [Google Scholar]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Mehta, P. Reducing the environmental impact of concrete. Concr. Int. 2001, 23, 61–66. [Google Scholar]
- Dassekpo, J.B.M.; Zha, X.; Zhan, J. Compressive strength performance of geopolymer paste derived from Completely Decomposed Granite (CDG) and partial fly ash replacement. Constr. Build. Mater. 2016, 138, 195–203. [Google Scholar] [CrossRef]
- Temuujin, J.; Williams, R.P.; van Riessen, A. Effect of mechanical activation of fly ash on the properties of geopolymer cured at ambient temperature. J. Mater. Process. Technol. 2009, 209, 5276–5280. [Google Scholar] [CrossRef]
- Bucher, K.; Grapes, R. Petrogenesis of Metamorphic Rocks, 8th ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 315–316. [Google Scholar]
- Danner, T.; Justnes, H.; Norden, G.; Østnor, T. Feasibility of Calcined Marl as an Alternative Pozzolanic Material. In Calcined Clays for Sustainable Concrete; Scrivener, K., Favier, A., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA, 2015; pp. 67–73. [Google Scholar]
- Rakhimova, N.R.; Rakhimov, R.Z.; Morozov, V.P.; Gaifullin, A.R.; Potapova, L.I.; Gubaidullina, A.M.; Osin, Y.N. Marl-based geopolymers incorporated with limestone: A feasibility study. J. Non-Cryst. Solids 2018, 492, 1–10. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Gracía-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
- Mikulčić, H.; von Berg, E.; Vujanović, M.; Priesching, P.; Perković, L.; Tatschl, R.; Duić, N. Numerical modeling of calcination reaction mechanism for cement production. Chem. Eng. Sci. 2012, 69, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Baronio, G.; Binda, L. Study of the pozzolanity of some bricks and clays. Constr. Build. Mater. 1997, 11, 41–46. [Google Scholar] [CrossRef]
- Buchwald, A.; Dombrowski, K.; Weil, M. The influence of calcium content on the performance of geopolymeric binder especially the resistance against acids. In Proceedings of the World Geopolymer, St. Quentin, France, 29 June–1 July 2005; pp. 35–39. [Google Scholar]
- Neville, A.M. Properties of Concrete, 4th ed.; Wiley: New York, NY, USA; Longmont, CO, USA; London, UK, 2002; pp. 840–844. [Google Scholar]
- Andersen, F.; Brečević, L. Infrared Spectra of Amorphous and Crystalline Calcium Carbonate. Acta Chem. Scand. 1991, 45, 1018–1024. [Google Scholar] [CrossRef]
- Mukherjee, S. Applied Mineralogy: Applications in Industry and Environment, 1st ed.; Springer Science & Business Media: Dordrecht, The Netherlands, 2011; pp. 65–73. [Google Scholar]
- Abd Rashid, R.; Shamsudin, R.; Abdul Hamid, M.A.; Jalar, A. Low temperature production of wollastonite from limestone and silica sand through solid-state reaction. J. Asian Ceram. Soc. 2014, 2, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Van Deventer, J. The Geopolymerisation of Alumino-silicate Minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Hardjito, D.; Cheak, C.; Ing, C. Strength and setting times of low calcium fly ash-based geopolymer mortar. Mod. Appl. Sci. 2008, 2, 3–11. [Google Scholar] [CrossRef]
- Al Bakri, A.M.M.; Kamarudin, H.; Omar, A.K.; Norazian, M.N.; Ruzaidi, C.M.; Rafiza, A.R. The Effect of Alkaline Activator Ratio on the Compressive Strength of Fly Ash-Based Geopolymers. Aust. J. Basic Appl. Sci. 2011, 5, 1916–1922. [Google Scholar]
- Liew, Y.; Kamarudin, H.; Al Bakri, A.M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Heah, C.Y. Processing and characterization of calcined kaolin cement powder. Constr. Build. Mater. 2012, 30, 794–802. [Google Scholar] [CrossRef]
- Abdul Rahim, R.H.; Azizli, K.A.; Man, Z.; Nuruddin, M.F. Effect of Curing Conditions on the Mechanical Properties of Fly Ash-Based Geopolymer without Sodium Silicate Solution. In Proceedings of the Third International Conference and Exhibition on Sustainable Energy and Advanced Material, Frankfurt am Main, Frankfurt am Main, Germany, 4–6 June 2013; pp. 1–6. [Google Scholar]
- Alshaaer, M.; El-Eswed, B.; Yousef, R.I.; Khalili, F.; Rahier, H. Development of functional geopolymers for water purification, and construction purposes. J. Saudi Chem. Soc. 2016, 20, S85–S92. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerisation and factors influencing its development: A review. J. Mater. Sci. 2007, 24, 729–746. [Google Scholar] [CrossRef]
- Elimbi, A.; Tchakoute, H.K.; Njopwouo, D. Effects of calcination temperature of kaolinite clays on the properties of geopolymer cements. Constr. Build. Mater. 2011, 25, 2805–2812. [Google Scholar] [CrossRef]
- Geman, R.; Tjondro, R.T.; Anggono, J.; Hardjito, D. Effects of Calcination Temperature of LUSI Mud on the Compressive Strength of Geopolymer Mortar. Adv. Mater. Res. 2013, 626, 224–228. [Google Scholar]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash. Part III: Effect of curing conditions on reaction and its graphical description. Fuel 2010, 89, 3185–3192. [Google Scholar] [CrossRef]
- Škvára, F.; Kopecký, L.; Šmilauer, V.; Bittnar, Z. Material and structural characterization of alkali activated low-calcium brown coal fly ash. J. Hazard. Mater. 2009, 168, 711–720. [Google Scholar] [CrossRef] [PubMed]














| Parameters | Calcined Marl (g) | Sodium hydroxide (SH) (g) | SH (Molarity) | Sodium silicate (SS) (g) | SS/SH Mass Ratios | Solid/liquid (S/L) Mass Ratios | Water (g) |
|---|---|---|---|---|---|---|---|
| Different NaOH molarities | 100 | 10, 20, 30, 40, and 50 | 2.5, 5, 8, 10, and 12 | 40 | 1.5 | 2 | 150, 140, 130, 120, and 110 |
| Different SS/SH mass ratios | 100 | 40 | 10 | 40 | 0.6, 1, 1.5, 2.3, and 3 | 2 | 120 |
| Different S/L mass ratios | 80, 85, 90, 100, and 110 | 40 | 10 | 40 | 1.5 | 1.5, 1.7, 1.8, 2, and 2.2 | 120 |
| SiO2 | Al2O3 | TiO2 | Fe2O3 | CaO | MgO | Na2O | K2O | MnO | P2O5 | SO3 | L.O.I | SUM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 46.95 | 5.88 | 0.60 | 0.85 | 28.0 | 0.73 | 0.20 | 0.39 | 0.13 | 1.75 | 0.70 | 13.73 | 99.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Habaak, G.; Askalany, M.; Abdel-Hakeem, M. Building up and Characterization of Calcined Marl-Based Geopolymeric Cement. Infrastructures 2018, 3, 22. https://0-doi-org.brum.beds.ac.uk/10.3390/infrastructures3030022
El-Habaak G, Askalany M, Abdel-Hakeem M. Building up and Characterization of Calcined Marl-Based Geopolymeric Cement. Infrastructures. 2018; 3(3):22. https://0-doi-org.brum.beds.ac.uk/10.3390/infrastructures3030022
Chicago/Turabian StyleEl-Habaak, Galal, Mohamed Askalany, and Mahmoud Abdel-Hakeem. 2018. "Building up and Characterization of Calcined Marl-Based Geopolymeric Cement" Infrastructures 3, no. 3: 22. https://0-doi-org.brum.beds.ac.uk/10.3390/infrastructures3030022