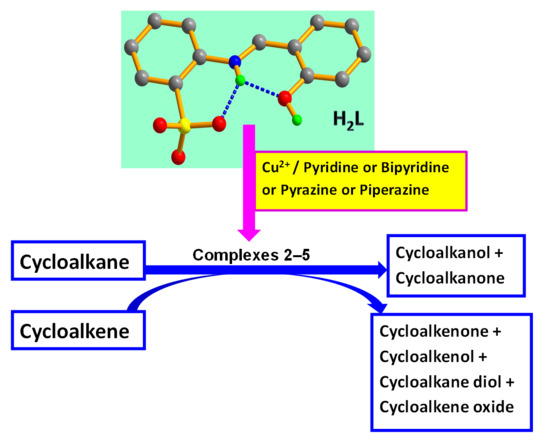
Syntheses, Structures, and Catalytic Hydrocarbon Oxidation Properties of N-Heterocycle-Sulfonated Schiff Base Copper(II) Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Syntheses and Characterization
2.2. Description of Crystal Structures of 1–5
2.2.1. Crystal Structure of H2L (1)
2.2.2. Crystal Structures of 2–5
2.2.3. Oxidation of Hydrocarbons
3. Materials and Methods
3.1. Synthesis of H2L (1)
3.2. Synthesis of [Cu(L)(py)(EtOH)] (2)
3.3. Synthesis of [Cu(L)(bipy)]·MeOH (3)
3.4. Synthesis of [Cu2(L)2(μ-pyr)(MeOH)2] (4)
3.5. Synthesis of [Cu2(L)2(μ-pip)(MeOH)2] (5)
3.6. Crystal Structure Determinations
3.7. Hydrocarbon Oxidation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hazra, S.; Martins, N.M.R.; Kuznetsov, M.L.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Flexibility and lability of a phenyl ligand in hetero-organometallic 3d metal–Sn(IV) compounds and their catalytic activity in Baeyer–Villiger oxidation of cyclohexanone. Dalton Trans. 2017, 46, 13364–13375. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Chakraborty, P.; Mohanta, S. Heterometallic Copper(II)–Tin(II/IV) Salts, Cocrystals, and Salt Cocrystals: Selectivity and Structural Diversity Depending on Ligand Substitution and the Metal Oxidation State. Cryst. Growth Des. 2016, 16, 3777–3790. [Google Scholar] [CrossRef]
- Hazra, S.; Meyrelles, R.; Charmier, A.J.; Rijo, P.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. N–H···O and N–H···Cl supported 1D chains of heterobimetallic CuII/NiII–SnIV cocrystals. Dalton Trans. 2016, 45, 17929–17938. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Titiš, J.; Valigura, D.; Boca, R.; Mohanta, S. Bis-phenoxido and bis-acetato bridged heteronuclear {CoIIIDyIII} single molecule magnets with two slow relaxation branches. Dalton Trans. 2016, 45, 7510–7520. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Bhattacharya, S.; Singh, M.K.; Carrella, L.; Rentschler, E.; Weyhermueller, T.; Rajaraman, G.; Mohanta, S. Syntheses, Structures, Magnetic Properties, and Density Functional Theory Magneto-Structural Correlations of Bis(μ-phenoxo) and Bis(μ-phenoxo)-μ-acetate/Bis(μ-phenoxo)-bis(μ-acetate) Dinuclear FeIIINiIICompounds. Inorg. Chem. 2013, 52, 12881–12892. [Google Scholar] [CrossRef]
- Hazra, S.; Sasmal, S.; Fleck, M.; Grandjean, F.; Sougrati, M.T.; Ghosh, M.; Harris, T.D.; Bonville, P.; Long, G.J.; Mohanta, S. Slow magnetic relaxation and electron delocalization in an S = 9/2iron(II/III) complex with two crystallographically inequivalent iron sites. J. Chem. Phys. 2011, 134, 174507. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Chemistry and Physics of Supramolecular Magnetic Materials. Acc. Chem. Res. 2000, 33, 647–657. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, H.; Chen, Y.; Ni, Z.-H.; Tian, L.; Jiang, J. Hydrogen-Bond Directed Cyanide-Bridged Molecular Magnets Derived from Polycyanidemetalates and Schiff Base Manganese(III) Compounds: Synthesis, Structures, and Magnetic Properties. Inorg. Chem. 2009, 48, 11215–11225. [Google Scholar] [CrossRef]
- Sasmal, S.; Hazra, S.; Kundu, P.; Majumder, S.; Aliaga-Alcalde, N.; Ruiz, E.; Mohanta, S. Magneto-Structural Correlation Studies and Theoretical Calculations of a Unique Family of Single End-to-End Azide-Bridged NiII4 Cyclic Clusters. Inorg. Chem. 2010, 49, 9517–9526. [Google Scholar] [CrossRef]
- Redshaw, C.; Elsegood, M.R.J.; Frese, J.W.A.; Ashby, S.; Chao, Y.; Mueller, A. Cellular uptake studies of two hexanuclear, carboxylate bridged, zinc ring structures using fluorescence microscopy. Chem. Commun. 2012, 48, 6627–6629. [Google Scholar] [CrossRef]
- Yang, X.; Jones, R.A. Anion Dependent Self-Assembly of “Tetra-Decker” and “Triple-Decker” Luminescent Tb(III) Salen Complexes. J. Am. Chem. Soc. 2005, 127, 7686–7687. [Google Scholar] [CrossRef]
- Liao, S.; Yang, X.; Jones, R.A. Self-Assembly of Luminescent Hexanuclear Lanthanide Salen Complexes. Cryst. Growth Des. 2012, 12, 970–974. [Google Scholar] [CrossRef]
- Wu, J.-Q.; Pan, L.; Li, Y.-G.; Liu, S.-R.; Li, Y.-S. Synthesis, Structural Characterization, and Olefin Polymerization Behavior of Vanadium(III) Complexes Bearing Tridentate Schiff Base Ligands. Organometallics 2009, 28, 1817–1825. [Google Scholar] [CrossRef]
- Bania, K.K.; Bharali, D.; Viswanathan, B.; Deka, R.C. Enhanced Catalytic Activity of Zeolite Encapsulated Fe(III)–Schiff-Base Complexes for Oxidative Coupling of 2-Napthol. Inorg. Chem. 2012, 51, 1657–1674. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.; Gnanadesikan, V.; Matsunaga, S.; Shibasaki, M. Heterobimetallic Transition Metal/Rare Earth Metal Bifunctional Catalysis: A Cu/Sm/Schiff Base Complex for Syn-Selective Catalytic Asymmetric Nitro-Mannich Reaction. J. Am. Chem. Soc. 2010, 132, 4925–4934. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, A.; Spielberg, E.T.; Görls, H.; Plass, W. Chiral Tetranuclear μ3-Alkoxo-Bridged Copper(II) Complex with 2 + 4 Cubane-Like Cu4O4 Core Framework and Ferromagnetic Ground State. Inorg. Chem. 2008, 47, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, C.; Elsegood, M.R.J. Novel organoaluminium (and gallium) carboxylate-bridgedring systems. Chem. Commun. 2001, 0, 2016–2017. [Google Scholar] [CrossRef]
- Puterová, Z.; Valentová, J.; Bojková, Z.; Kožíšek, J.; Devínsky, F. Synthesis, crystal structure and antiradical effect of copper(II) Schiff base complexes containing five-, six- and unusual seven-membered rings. Dalton Trans. 2011, 40, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Mukherjee, S.; da Silva, M.F.; Pombeiro, A.J. A cyclic tetranuclear cuboid type copper(II) complex doubly supported by cyclohexane-1,4-dicarboxylate: Molecular and supramolecular structure and cyclohexane oxidation activity. RSC Adv. 2014, 4, 48449–48457. [Google Scholar] [CrossRef]
- Hazra, S.; Karmakar, A.; Guedes da Silva, M.F.C.; Dlhán, L.; Boca, R.; Pombeiro, A.J.L. Sulfonated Schiff base dinuclear and polymeric copper(II) complexes: Crystal structures, magnetic properties and catalytic application in Henry reaction. New J. Chem. 2015, 39, 3424–3434. [Google Scholar] [CrossRef]
- Hazra, S.; Karmakar, A.; Guedes da Silva, M.F.C.; Dlhán, L.; Boca, R.; Pombeiro, A.J.L. Dinuclear based polymeric copper(II) complexes derived from a Schiff base ligand: Effect of secondary bridging moieties on geometrical orientations and magnetic properties. Inorg. Chem. Commun. 2014, 46, 113–117. [Google Scholar] [CrossRef]
- Hazra, S.; Guedes da Silva, M.F.C.; Karmakar, A.; Pombeiro, A.J.L. 1D hacksaw chain bipyridine–sulfonate Schiff base-dicopper(II) as a host for variable solvent guests. RSC Adv. 2015, 5, 28070–28079. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; Martins, L.M.; Hazra, S.; Pombeiro, A.J. Catalytic oxidation of cyclohexane with hydrogen peroxide and a tetracopper(II) complex in an ionic liquid. Compt. Rend. Chim. 2015, 18, 758–765. [Google Scholar] [CrossRef]
- Hazra, S.; Martins, L.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Sulfonated Schiff base copper(II) complexes as efficient and selective catalysts in alcohol oxidation: Syntheses and crystal structures. RSC Adv. 2015, 5, 90079–90088. [Google Scholar] [CrossRef]
- Martins, L.M.; Hazra, S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. A sulfonated Schiff base dimethyltin(IV) coordination polymer: Synthesis, characterization and application as a catalyst for ultrasound- or microwave-assisted Baeyer–Villiger oxidation under solvent-free conditions. RSC Adv. 2016, 6, 78225–78233. [Google Scholar] [CrossRef]
- Hazra, S.; Paul, A.; Sharma, G.; Koch, B.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Sulfonated Schiff base Sn(IV) complexes as potential anticancer agents. J. Inorg. Biochem. 2016, 162, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Martins, L.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Sulfonated Schiff base dimeric and polymeric copper(II) complexes: Temperature dependent synthesis, crystal structure and catalytic alcohol oxidation studies. Inorg. Chim. Acta 2017, 455, 549–556. [Google Scholar] [CrossRef]
- Hazra, S.; Ribeiro, A.P.C.; Guedes da Silva, M.F.C.; Nieto de Castro, C.A.; Pombeiro, A.J.L. Syntheses and crystal structures of benzene-sulfonate and -carboxylate copper polymers and their application in the oxidation of cyclohexane in ionic liquid under mild conditions. Dalton Trans. 2016, 45, 13957–13968. [Google Scholar] [CrossRef]
- Paul, A.; Hazra, S.; Sharma, G.; Guedes da Silva, M.F.C.; Koch, B.; Pombeiro, A.J.L. Unfolding biological properties of a versatile dicopper(II) precursor and its two mononuclear copper(II) derivatives. J. Inorg. Biochem. 2017, 174, 25–36. [Google Scholar] [CrossRef]
- Othmer, D.F. Kirk-Othmer Concise Encyclopedia of Chemical Technology, 5th ed.; 2nd Volume Set; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Sutradhar, M.; Shvydkiy, N.V.; Guedes da Silva, M.F.C.; Kirillova, M.V.; Kozlov, Y.N.; Pombeiro, A.J.L.; Shul’pin, G.B. A new binuclear oxovanadium(V) complex as a catalyst in combination with pyrazinecarboxylic acid(PCA) for efficient alkane oxygenation by H2O2. Dalton Trans. 2013, 42, 11791–11803. [Google Scholar] [CrossRef]
- Sutradhar, M.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. A Hexanuclear Mixed-Valence Oxovanadium(IV,V) Complex as a Highly Efficient Alkane Oxidation Catalyst. Inorg. Chem. 2012, 51, 11229–11232. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.S.; Labinger, J.A.; Bercaw, J.E. Homogeneous Oxidation of Alkanes by Electrophilic Late Transition Metals. Angew. Chem. Int. Ed. 1998, 37, 2180–2192. [Google Scholar] [CrossRef]
- Mokaya, R.; Poliakoff, M. A cleaner way to nylon? Nature 2005, 437, 1243–1244. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, F. Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Schuchardt, U.; Cardoso, D.; Sercheli, R.; Pereira, R.; da Cruz, R.S.; Guerreiro, M.C.; Mandelli, D.; Spinacé, E.V.; Pires, E.L. Cyclohexane oxidation continues to be a challenge. Appl. Catal. A: Gen. 2001, 211, 1–17. [Google Scholar] [CrossRef]
- Slaughter, L.M.; Collman, J.P.; Eberspacher, T.A.; Brauman, J.I. Radical Autoxidation and Autogenous O2 Evolution in Manganese–Porphyrin Catalyzed Alkane Oxidations with Chlorite. Inorg. Chem. 2004, 43, 5198–5204. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.S.; Burgess, K. Metal-Catalyzed Epoxidations of Alkenes with Hydrogen Peroxide. Chem. Rev. 2003, 103, 2457–2474. [Google Scholar] [CrossRef] [PubMed]
- Grigoropoulou, G.; Clark, J.H.; Elings, J.A. Recent developments on the epoxidation of alkenes using hydrogen peroxide as an oxidant. Green Chem. 2003, 5, 1–7. [Google Scholar] [CrossRef]
- Muzart, J. Pd-mediated epoxidation of olefins. J. Mol. Catal. A 2007, 276, 62–72. [Google Scholar] [CrossRef]
- Díaz-Requejo, M.M.; Pérez, P.J. Coinage Metal Catalyzed C–H Bond Functionalization of Hydrocarbons. Chem. Rev. 2008, 108, 3379–3394. [Google Scholar] [CrossRef]
- Crabtree, R.H. Editorial: Introduction to Selective Functionalization of C–H Bonds. Chem. Rev. 2010, 110, 575. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Pombeiro, A.J.L. Homo- and heterometallic polynuclear transition metal catalysts for alkane C–H bonds oxidative functionalization: Recent advances. Coord. Chem. Rev. 2018, 355, 199–222. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Half-Sandwich Scorpionate Vanadium, Iron and Copper Complexes: Synthesis and Application in the Catalytic Peroxidative Oxidation of Cyclohexane under Mild Conditions. Adv. Synth. Catal. 2008, 350, 706–716. [Google Scholar] [CrossRef]
- Silva, T.F.; Mishra, G.S.; da Silva, M.F.; Wanke, R.; Martins, L.M.; Pombeiro, A.J. CuII complexes bearing the 2,2,2-tris(1-pyrazolyl)ethanol or 2,2,2-tris(1-pyrazolyl)ethyl methanesulfonate scorpionates. X-Ray structural characterization and application in the mild catalyticperoxidative oxidation of cyclohexane. Dalton Trans. 2009, 9207–9215. [Google Scholar] [CrossRef]
- Da Silva, J.A.L.; da Silva, J.J.R.F.; Pombeiro, A.J.L. Oxovanadium complexes in catalytic oxidations. Coord. Chem. Rev. 2011, 255, 2232–2248. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kopylovich, M.N.; Kirillova, M.V.; Haukka, M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Multinuclear Copper Triethanolamine Complexes as Selective Catalysts for the Peroxidative Oxidation of Alkanes under Mild Conditions. Angew. Chem. Int. Ed. 2005, 44, 4345–4349. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.M.; Kopylovich, M.N.; Kirillova, M.V.; Karabach, Y.Y.; Haukka, M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Mild Peroxidative Oxidation of Cyclohexane Catalyzed by Mono-, Di-, Tri-, Tetra- and Polynuclear Copper Triethanolamine Complexes. Adv. Synth. Catal. 2006, 348, 159–174. [Google Scholar] [CrossRef]
- Nicola, C.D.; Karabach, Y.Y.; Kirillov, A.M.; Monari, M.; Pandolfo, L.; Pettinari, C.; Pombeiro, A.J.L. Supramolecular Assemblies of Trinuclear Triangular Copper(II) Secondary Building Units through Hydrogen Bonds. Generation of Different Metal−Organic Frameworks, Valuable Catalysts for Peroxidative Oxidation of Alkanes. Inorg. Chem. 2007, 46, 221–230. [Google Scholar] [CrossRef]
- Kuznetsov, M.; Pombeiro, A.J.L. Radical Formation in the [MeReO3]-Catalyzed Aqueous Peroxidative Oxidation of Alkanes: A Theoretical Mechanistic Study. Inorg. Chem. 2009, 48, 307–318. [Google Scholar] [CrossRef]
- Roy, P.; Manassero, M. Tetranuclear copper(II)–Schiff-base complexes as active catalysts for oxidation of cyclohexane and toluene. Dalton Trans. 2010, 39, 1539–1545. [Google Scholar] [CrossRef]
- Dronova, M.S.; Bilyachenko, A.N.; Yalymov, A.I.; Kozlov, Y.N.; Shul’pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; Levitsky, M.M.; Shubina, E.S.; Shul’pin, G.B. Solvent-controlled synthesis of tetranuclear cage-like copper(II) silsesquioxanes. Remarkable features of the cage structures and their high catalytic activity in oxidation with peroxides. Dalton Trans. 2014, 43, 872–882. [Google Scholar] [CrossRef]
- Rocha, B.G.M.; Kuznetsov, M.L.; Kozlov, Y.N.; Pombeiro, A.J.L.; Shul’pin, G.B. Simple soluble Bi(III) salts as efficient catalysts for the oxidation of alkanes with H2O2. Catal. Sci. Technol. 2015, 5, 2174–2187. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Gradinaru, J.; Kozlov, Y.N. Alkane hydroperoxidation with peroxides catalyzed by copper complexes. Org. Biomol. Chem. 2003, 1, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Süss-Fink, G.; Gonzalez, L.; Shul’pin, G.B. Alkane oxidation with hydrogen peroxide catalyzed homogeneously by vanadium-containing polyphosphomolybdates. Appl. Catal. A 2001, 217, 111–117. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kozlov, Y.N.; Shul’pina, L.S.; Lyakin, O.Y.; Kirillov, A.M.; Talsi, E.P.; Pombeiro, A.J.L.; Shul’pin, G.B. Remarkably fast oxidation of alkanes by hydrogen peroxide catalyzed by a tetracopper(II) triethanolaminate complex: Promoting effects of acid co-catalysts and water, kinetic and mechanistic features. J. Catal. 2009, 268, 26–38. [Google Scholar] [CrossRef]
- Costas, M.; Mehn, M.P.; Jensen, M.P.; Que, L., Jr. Dioxygen Activation at Mononuclear Nonheme Iron Active Sites: Enzymes, Models, and Intermediates. Chem. Rev. 2004, 104, 939–986. [Google Scholar] [CrossRef] [PubMed]
- Costas, M.; Chen, K.; Que, L., Jr. Biomimetic nonheme iron catalysts for alkane hydroxylation. Coord. Chem. Rev. 2000, 200–202, 517–544. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Transition Metals for Organic Synthesis, 2nd ed.; Bellera, M., Bolm, C., Eds.; Wiley-VCH: New York, NY, USA, 2004; p. 215. [Google Scholar]
- Shul’pin, G.B.; Nizova, G.V. Formation of alkyl peroxides in oxidation of alkanes by H2O2 catalyzed by transition metal complexes. React. Kinet. Catal. Lett. 1992, 48, 333–338. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Nizova, G.V.; Süss-Fink, G.; Stanislas, S.; Kitaygorodskiy, A.; Kulikova, V.S. Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”. Part 12.1Main features, kinetics and mechanism of alkane hydroperoxidation. J. Chem. Soc. Perkin Trans. 2001, 2, 1351–1371. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Matthes, M.G.; Romakh, V.B.; Barbosa, M.I.F.; Aoyagi, J.L.T.; Mandelli, D. Oxidations by the system “hydrogen peroxide–[Mn2L2O3][PF6]2 (L=1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid”. Part 10: Co-catalytic effect of different carboxylic acids in the oxidation of cyclohexane, cyclohexanol, and acetone. Tetrahedron 2008, 64, 2143–2152. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A: Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B. C–H functionalization: Thoroughly tuning ligands at a metal ion, a chemist can greatly enhance catalyst’s activity and selectivity. Dalton Trans. 2013, 42, 12794–12818. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Selectivity enhancement in functionalization of C–H bonds: A review. Org. Biomol. Chem. 2010, 8, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Kodera, M.; Shimakoshi, H.; Kano, K. First example of a rigid (µ-oxo-di-µ-acetato) diiron(III) complex with 1,2-bis[2-di(2-pyridyl)methyl-6-pyridyl]ethane; its efficient catalysis for functionalization of alkanes. Chem. Commun. 1996, 0, 1737–1738. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Müller, W. Selective functionalization of hydrocarbons. 6. Mechanistic and preparative studies on the regio- and stereoselective paraffin hydroxylation with peracids. J. Org. Chem. 1985, 50, 4609–4615. [Google Scholar] [CrossRef]
- Mikami, Y.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Superior Performance of Fe(BTC) With Respect to Other Metal-Containing Solids in the N-Hydroxyphthalimide-Promoted Heterogeneous Aerobic Oxidation of Cycloalkanes. ChemCatChem 2013, 5, 1964–1970. [Google Scholar] [CrossRef]
- Theyssen, N.; Leitner, W. Selective oxidation of cyclooctane to cyclootanone with molecular oxygen in the presence of compressed carbon dioxide. Chem. Commun. 2002, 410–411. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Primo, A.; Concepcion, P.; Alvaro, M.; Garcia, H. Doped Graphene as a Metal-Free Carbocatalyst for the Selective Aerobic Oxidation of Benzylic Hydrocarbons, Cyclooctane and Styrene. Chem. Eur. J. 2013, 19, 7547–7554. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, W.; Ma, D.; Liu, Z. Metallo-deuteroporphyrins as catalysts for the oxidation of cyclohexane with air in the absence of additives and solvents. Catal. Commun. 2008, 10, 83–85. [Google Scholar] [CrossRef]
- Halvagar, M.R.; Solntsev, P.V.; Lim, H.; Hedman, B.; Hodgson, K.O.; Solomon, E.I.; Cramer, C.J.; Tolman, W.B. Hydroxo-Bridged Dicopper(II,III) and -(III,III) Complexes: Models for Putative Intermediates in Oxidation Catalysis. J. Am. Chem. Soc. 2014, 136, 7269–7272. [Google Scholar] [CrossRef]
- Haack, P.; Kärgel, A.; Greco, C.; Dokic, J.; Braun, B.; Pfaff, F.F.; Mebs, S.; Ray, K.; Limberg, C. Access to a CuII–O–CuII Motif: Spectroscopic Properties, Solution Structure, and Reactivity. J. Am. Chem. Soc. 2013, 135, 16148–16151. [Google Scholar] [CrossRef]
- Mirica, L.M.; Ottenwaelder, X.; Stack, T.D.P. Structure and Spectroscopy of Copper–Dioxygen Complexes. Chem. Rev. 2004, 104, 1013–1046. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, R.L.; Rosenzweig, A.C. Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane. Nature 2005, 434, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Mahmudov, K.T.; Liu, C.-M.; Pombeiro, A.J.L. Trinuclear CuII Structural Isomers: Coordination, Magnetism, Electrochemistry and Catalytic Activity towards the Oxidation of Alkanes. Eur. J. Inorg. Chem. 2015, 23, 3959–3969. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Shul’pin, G.B. Pyrazinecarboxylic acid and analogs: Highly efficient co-catalysts in the metal–complex-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2013, 257, 732–754. [Google Scholar] [CrossRef]
- Bruker. APEX2 and SAINT; Bruker, AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. Sect. A: Found. Crystallogr. 1990, A46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]








| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| In the Organic Moiety H2L (or L2−) | |||||
| C−Nimino | 1.299(4) 1.294(4) | 1.303(4) | 1.292(3) | 1.301(3) | 1.309(5) |
| Caromatic−Nimino | 1.433(4) 1.438(4) | 1.429(4) | 1.434(3) | 1.426(3) | 1.429(5) |
| Coplanarity of aromatic rings (°) | 8.41 10.98 | 58.19 | 62.01 | 51.04 | 51.81 |
| Surrounding the Copper Atom | |||||
| Cu−Nimino | - | 1.976(2) | 1.9837(19) | 1.9769(17) | 1.975(3) |
| Cu−Osulfonato | - | 2.014(2) | 2.3789(18) | 1.9733(16) | 2.019(3) |
| Cu−Ophenoxido | - | 1.898(2) | 1.8942(18) | 1.8770(16) | 1.900(3) |
| Cu−Ncoligand | - | 2.015(3) | 2.009(2) 2.061(2) | 2.0709(17) | 2.004(3) |
| Cu−Ocoligand | - | 2.290(2) | - | 2.3760(18) | 2.303(3) |
| ∠N−Cu−N | - | 177.98(10) | 79.71(8) 102.71(8) 170.85(8) | 174.06(7) | 177.57(14) |
| ∠Osulfonato−Cu− Ophenoxido | - | 157.30(9) | 113.95(8) | 158.97(8) | 151.07(14) |
| Cu coordination environment | - | N2O3 | N3O2 | N2O3 | N2O3 |
| τ5 | - | 0.35 | 0.27 | 0.25 | 0.44 |
| Cu···Cu (minimum) | - | 7.541 | 8.110 | 6.919 | 6.782 |
| No Additive | py | HNO3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Time (min) | Yield b (%) | Selectivity c | TON d | Yield b (%) | Selectivity c | TON d | Yield b (%) | Selectivity c | TON d |
| 1 | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - | 0 |
| 2 | 1 | 3 | 0:100 | 8 | ||||||
| 3 | 3 | 9 | 1:99 | 23 | ||||||
| 4 | 4 | 3 | 1:99 | 8 | 14 | 4:96 | 35 | 13 | 1:99 | 31 |
| 5 | 5.5 | 23 | 7:93 | 58 | ||||||
| 6 | 7 | 26 | 11:89 | 65 | ||||||
| 7 | 9 | 8 | 3:97 | 20 | 26 | 14:86 | 65 | |||
| 8 | 10 | 26 | 21:79 | 65 | 14 | 2:98 | 35 | |||
| 9 | 15 | 14 | 5:95 | 35 | 26 | 24:76 | 65 | |||
| 10 | 30 | 18 | 6:94 | 45 | 26 | 28:72 | 65 | 17 | 3:97 | 42 |
| 11 | 60 | 20 | 10:90 | 50 | 26 | 27:73 | 65 | |||
| 12 | 90 | 20 | 17:83 | 50 | 26 | 29:71 | 65 | 19 | 5:95 | 47 |
| 13 | 2 × 60 | 20 | 20:80 | 50 | 26 | 34:66 | 65 | 20 | 7:93 | 50 |
| 14 | 4 × 60 | 20 | 23:77 | 50 | 26 | 37:63 | 65 | 22 | 10:90 | 56 |
| 15 | 5.5 × 60 | 23 | 10:90 | 58 | ||||||
| 16 | 8 × 60 | 20 | 27:73 | 51 | 26 | 39:61 | 65 | 25 | 11:89 | 63 |
| 17 | 10 × 60 | 27 | 11:89 | 68 | ||||||
| 18 | 16 × 60 | 30 | 13:87 | 74 | ||||||
| 19 | 21.5 × 60 | 26 | 20:80 | 65 | ||||||
| 20 | 24 × 60 | 20 | 31:69 | 50 | 26 | 53:47 | 65 | 25 | 20:80 | 62 |
| 21 | 29.5 × 60 | 20 | 23:77 | 50 | ||||||
| 22 | 32 × 60 | 20 | 36:64 | 51 | 26 | 54:46 | 65 | 18 | 25:75 | 46 |
| No Additive | py | HNO3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Time(min) | Yield b (%) | Selectivity c | TON d | Yield b (%) | Selectivity c | TON d | Yield b (%) | Selectivity c | TON d |
| 1 | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - | 0 |
| 2 | 1 | 1 | 0:100 | 3 | ||||||
| 3 | 3 | 5 | 3:97 | 13 | ||||||
| 4 | 4 | 7 | 4:96 | 17 | ||||||
| 5 | 6 | 13 | 5:95 | 33 | ||||||
| 6 | 8 | 18 | 7:93 | 45 | ||||||
| 7 | 10 | 23 | 8:92 | 58 | ||||||
| 8 | 15 | 23 | 16:84 | 58 | 3 | 0:100 | 8 | |||
| 9 | 30 | 6 | 2:98 | 15 | 23 | 21:79 | 58 | 6 | 0:100 | 15 |
| 10 | 60 | 7 | 3:97 | 18 | 23 | 26:74 | 58 | 8 | 1:98 | 19 |
| 11 | 90 | 23 | 27:73 | 58 | 10 | 1:98 | 25 | |||
| 12 | 2 × 60 | 9 | 3:97 | 23 | 23 | 29:71 | 58 | 12 | 2:98 | 31 |
| 13 | 3 × 60 | 19 | 47 | |||||||
| 14 | 4 × 60 | 11 | 9:91 | 28 | 23 | 34:66 | 58 | 30 | 3:97 | 76 |
| 15 | 8 × 60 | 13 | 23:77 | 32 | 23 | 40:60 | 58 | 26 | 23:77 | 64 |
| 16 | 10 × 60 | 13 | 28:72 | 31 | ||||||
| 17 | 24 × 60 | 13 | 34:66 | 32 | 23 | 56:44 | 58 | 15 | 34:66 | 37 |
| 18 | 32 × 60 | 13 | 39:61 | 31 | 23 | 61:39 | 58 | 13 | 39:61 | 32 |
| No Additive | py | HNO3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Time(h) | Yield b (%) | Selectivity c | TON d | Yield b (%) | Selectivity c | TON d | Yield b (%) | Selectivity c | TON d |
| 1 | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - | 0 |
| 2 | 1 | 3 | 0:100 | 8 | ||||||
| 3 | 3 | 9 | 3:97 | 23 | ||||||
| 4 | 4 | 15 | 12:88 | 38 | ||||||
| 5 | 5.5 | 19 | 20:80 | 48 | ||||||
| 6 | 7 | 21 | 22:78 | 53 | ||||||
| 7 | 9 | 23 | 25:75 | 58 | ||||||
| 8 | 10 | 24 | 26:74 | 60 | ||||||
| 9 | 15 | 24 | 27:73 | 60 | 3 | 0:100 | 7.5 | |||
| 10 | 30 | 24 | 29:71 | 60 | 8 | 0:100 | 20 | |||
| 11 | 60 | 24 | 30:70 | 60 | 16 | 0:100 | 40 | |||
| 12 | 90 | 22 | 1:98 | 55 | ||||||
| 13 | 2 × 60 | 6 | 0:100 | 16 | 24 | 32:68 | 60 | 31 | 2:98 | 78 |
| 14 | 4 × 60 | 8 | 2:98 | 20 | 24 | 34:66 | 60 | 35 | 2:98 | 88 |
| 15 | 8 × 60 | 8 | 4:96 | 21 | 24 | 39:61 | 60 | 26 | 5:95 | 65 |
| 16 | 24 × 60 | 8 | 32:68 | 21 | 24 | 49:51 | 60 | 20 | 38:62 | 50 |
| 17 | 32 × 60 | 8 | 36:64 | 21 | 24 | 52:48 | 60 | 17 | 33:67 | 43 |
| No Additive | py | HNO3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Time(min) | Yield b (%) | Selectivity c | TON d | Yield b (%) | Selectivity c | TON d | Yield b (%) | Selectivity c | TON d |
| 1 | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - | 0 |
| 2 | 1 | 1 | 0:100 | 3 | ||||||
| 3 | 3 | 5 | 0:100 | 13 | ||||||
| 4 | 4 | 9 | 1:99 | 23 | ||||||
| 5 | 5.5 | 13 | 8:92 | 33 | ||||||
| 6 | 7 | 17 | 12:88 | 43 | ||||||
| 7 | 9 | 20 | 15:85 | 50 | ||||||
| 8 | 10 | 21 | 17:83 | 53 | ||||||
| 9 | 15 | 23 | 19:81 | 58 | ||||||
| 10 | 30 | 23 | 26:74 | 58 | ||||||
| 11 | 60 | 23 | 28:72 | 58 | 7 | 0:100 | 19 | |||
| 12 | 90 | 23 | 30:70 | 58 | ||||||
| 13 | 2 × 60 | 4 | 0:100 | 11 | 23 | 31:69 | 58 | 14 | 2:98 | 36 |
| 14 | 4 × 60 | 7 | 2:98 | 17 | 23 | 36:64 | 58 | 27 | 2:98 | 68 |
| 15 | 8 × 60 | 7 | 3:97 | 18 | 23 | 43:57 | 58 | 23 | 4:96 | 57 |
| 16 | 10 × 60 | 24 | 6:94 | 59 | ||||||
| 17 | 24 × 60 | 7 | 26:74 | 17 | 23 | 53:47 | 58 | 13 | 32:68 | 32 |
| 18 | 32 × 60 | 7 | 33:67 | 18 | 23 | 53:47 | 58 | 14 | 37:63 | 35 |
| Entry | Time (h) | Substrate | Yield b, % | Selectivity c | TON d |
|---|---|---|---|---|---|
| 1 | 2 | Cyclohexane | 20 | 22:78 | 50 |
| 2 | 4 | 20 | 24:76 | 50 | |
| 3 | 8 | 20 | 27:73 | 50 | |
| 4 | 24 | 20 | 32:68 | 50 | |
| 5 | 32 | 20 | 33:67 | 50 | |
| 6 | 2 | Cyclohexene | 15 | 8:36:44:4:8 | 50 |
| 7 | 4 | 15 | 7:40:39:5:9 | 50 | |
| 8 | 8 | 15 | 6:45:36:5:8 | 50 | |
| 9 | 24 | 15 | 1:49:34:8:8 | 50 | |
| 10 | 32 | 15 | 0:48:32:10:9 | 50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazra, S.; Rocha, B.G.M.; Guedes da Silva, M.F.C.; Karmakar, A.; Pombeiro, A.J.L. Syntheses, Structures, and Catalytic Hydrocarbon Oxidation Properties of N-Heterocycle-Sulfonated Schiff Base Copper(II) Complexes. Inorganics 2019, 7, 17. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7020017
Hazra S, Rocha BGM, Guedes da Silva MFC, Karmakar A, Pombeiro AJL. Syntheses, Structures, and Catalytic Hydrocarbon Oxidation Properties of N-Heterocycle-Sulfonated Schiff Base Copper(II) Complexes. Inorganics. 2019; 7(2):17. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7020017
Chicago/Turabian StyleHazra, Susanta, Bruno G. M. Rocha, M. Fátima C. Guedes da Silva, Anirban Karmakar, and Armando J. L. Pombeiro. 2019. "Syntheses, Structures, and Catalytic Hydrocarbon Oxidation Properties of N-Heterocycle-Sulfonated Schiff Base Copper(II) Complexes" Inorganics 7, no. 2: 17. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7020017