An Optical Power Limiting and Ultrafast Photophysics Investigation of a Series of Multi-Branched Heavy Atom Substituted Fluorene Molecules
Abstract
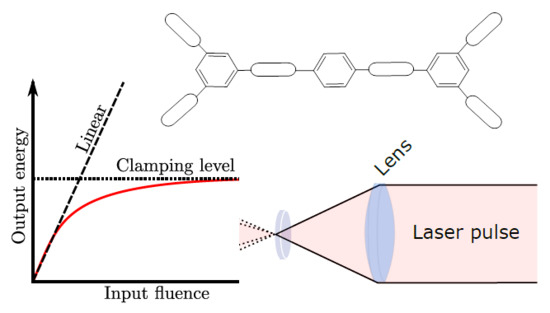
:1. Introduction
2. Results
2.1. Luminescence and Linear Transmittance
2.2. Wavelength-Scanned OPL Measurements
2.3. Excited State Absorption
Ultra-Fast Transient Absorption Measurements
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.1.1. Synthesis of “A”
4.1.2. Synthesis of “B”
4.1.3. Synthesis of the Platinum Complex “C”
4.2. Optical Absorbance and Luminescence
4.3. Wavelength-Scanned OPL Measurements
4.4. Excited State Absorption
4.5. Ultra-Fast Transient Absorption Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dini, D.; Calvete, M.J.F.; Hanack, M. Nonlinear Optical Materials for the Smart Filtering of Optical Radiation. Chem. Rev. 2016, 116, 13043–13233. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Shelton, A.H.; Kim, K.Y.; Schanze, K.S. Organoplatinum chromophores for application in high-performance nonlinear absorption materials. ACS Appl. Mater. Interfaces 2011, 3, 3225–3238. [Google Scholar] [CrossRef] [PubMed]
- Bretonnière, Y.; Andraud, C. Chromophores for Optical Power Limiting. In Photosensitizers in Medicine, Environment, and Security; Nyokong, T., Ahsen, V., Eds.; Springer: Heidelberg, The Netherlands, 2012; pp. 619–654. ISBN 978-90-481-3872-2. [Google Scholar]
- Zhou, G.J.; Wong, W.Y.; Lin, Z.; Ye, C. White metallopolyynes for optical limiting/transparency trade-off optimization. Angew. Chem. Int. Ed. 2006, 45, 6189–6193. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Olivier, J.-H.; Yoo, H.; Polizzi, N.F.; Park, J.; Rawson, J.; Therien, M.J. Molecular Road Map to Tuning Ground State Absorption and Excited State Dynamics of Long-Wavelength Absorbers. J. Am. Chem. Soc. 2017, 139, 16946–16958. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Shelton, A.H.; Drobizhev, M.; Makarov, N.; Rebane, A.; Schanze, K.S. Optimizing Simultaneous Two-Photon Absorption and Transient Triplet-Triplet Absorption in Platinum Acetylide Chromophores. J. Phys. Chem. A 2010, 114, 7003–7013. [Google Scholar] [CrossRef]
- Triadon, A.; Grelaud, G.; Richy, N.; Mongin, O.; Moxey, G.J.; Dixon, I.M.; Yang, X.; Wang, G.; Barlow, A.; Rault-berthelot, J.; et al. Linear and Third-Order Nonlinear Optical Properties of Fe(η5-C5Me5)(κ2-dppe)- and trans-Ru(κ2-dppe)2-Alkynyl Complexes Containing 2-Fluorenyl End Groups. Organometallics 2018, 37, 2245–2262. [Google Scholar] [CrossRef]
- Tian, Z.; Yang, X.; Liu, B.; Zhong, D.; Zhou, G.; Wong, W.-Y. New heterobimetallic Au(I)–Pt(II) polyynes achieving a good trade-off between transparency and optical power limiting performance. J. Mater. Chem. C 2018, 6, 11416–11426. [Google Scholar] [CrossRef]
- Girardot, C.; Cao, B.; Mulatier, J.; Baldeck, P.L.; Chauvin, J. Ruthenium(II) Complexes for Two-Photon Absorption-Based Optical Power Limiting. ChemPhysChem 2008, 9, 1531–1535. [Google Scholar] [CrossRef]
- Zhou, G.J.; Wong, W.Y.; Ye, C.; Lin, Z. Optical power limiters based on colorless di-, oligo-, and polymetallaynes: Highly transparent materials for eye protection devices. Adv. Funct. Mater. 2007, 17, 963–975. [Google Scholar] [CrossRef]
- Lanoë, P.H.; Gallavardin, T.; Dupin, A.; Maury, O.; Baldeck, P.L.; Lindgren, M.; Monnereau, C.; Andraud, C. Influence of bromine substitution pattern on the singlet oxygen generation efficiency of two-photon absorbing chromophores. Org. Biomol. Chem. 2012, 10, 6275–6278. [Google Scholar] [CrossRef]
- Gallavardin, T.; Armagnat, C.; Maury, O.; Baldeck, P.L.; Lindgren, M.; Monnereau, C.; Andraud, C. An improved singlet oxygen sensitizer with two-photon absorption and emission in the biological transparency window as a result of ground state symmetry-breaking. Chem. Commun. 2012, 48, 1689–1691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wei, G.; Zhu, W.; Ke, F.; Guang, S.; Zhang, F.; Xu, H. Controllable preparation and near infrared optical limiting properties of fluorene-containing polyacetylenes. J. Appl. Polym. Sci. 2018, 135, 46100. [Google Scholar] [CrossRef]
- Zheng, Q.; Gupta, S.K.; He, G.S.; Tan, L.; Prasad, P.N. Synthesis, Characterization, Two-Photon Absorption, and Optical Limiting Properties of Ladder-Type Oligo-p-phenylene-Cored Chromophores. Adv. Funct. Mater. 2008, 18, 2770–2779. [Google Scholar] [CrossRef]
- Morel, Y.; Irimia, A.; Najechalski, P.; Kervella, Y.; Stephan, O.; Baldeck, P.L.; Andraud, C. Two-photon absorption and optical power limiting of bifluorene molecule. J. Chem. Phys. 2001, 114, 5391–5396. [Google Scholar] [CrossRef]
- Kurhuzenkau, S.A.; Yezabel, M.; Gomez, C.; Bel, K.D.; Shaydyuk, Y.O.; Hagan, D.J.; Stryland, E.W.; Van Sissa, C.; Bondar, M.V.; Painelli, A. Electronic Nature of Nonlinear Optical Properties of a Symmetrical Two-Photon Absorbing Fluorene Derivative: Experimental Study and Theoretical Modeling. J. Phys. Chem. C 2018, 122, 5664–5672. [Google Scholar] [CrossRef]
- Rogers, J.E.; Slagle, J.E.; Krein, D.M.; Burke, A.R.; Hall, B.C.; Fratini, A.; McLean, D.G.; Fleitz, P.A.; Cooper, T.M.; Drobizhev, M.; et al. Platinum acetylide two-photon chromophores. Inorg. Chem. 2007, 46, 6483–6494. [Google Scholar] [CrossRef]
- Li, C.; Yang, K.; Feng, Y.; Su, X.; Yang, J.; Jin, X.; Shui, M.; Wang, Y.; Zhang, X.; Song, Y.; et al. Investigation of Two-Photon Absorption Induced Excited State Absorption in a Fluorenyl-Based Chromophore. J. Phys. Chem. B 2009, 113, 15730–15733. [Google Scholar] [CrossRef]
- Lin, T.-C.; Chen, Y.-F.; Hu, C.-L.; Hsu, C.-S. Two-photon absorption and optical power limiting properties in femtosecond regime of novel multi-branched chromophores based on tri-substituted olefinic scaffolds. J. Mater. Chem. 2009, 19, 7075–7080. [Google Scholar] [CrossRef]
- Chateau, D.; Chaput, F.; Lopes, C.; Lindgren, M.; Brännlund, C.; Öhgren, J.; Djourelov, N.; Nedelec, P.; Desroches, C.; Eliasson, B.; et al. Silica hybrid sol-gel materials with unusually high concentration of ptorganic molecular guests: Studies of luminescence and nonlinear absorption of light. ACS Appl. Mater. Interfaces 2012, 4, 2369–2377. [Google Scholar] [CrossRef]
- Zieba, R.; Desroches, C.; Chaput, F.; Carlsson, M.; Eliasson, B.; Lopes, C.; Lindgren, M.; Parola, S. Preparation of functional hybrid glass material from platinum(II) complexes for broadband nonlinear absorption of light. Adv. Funct. Mater. 2009, 19, 235–241. [Google Scholar] [CrossRef]
- Chateau, D.; Liotta, A.; Lundén, H.; Lerouge, F.; Chaput, F.; Krein, D.; Cooper, T.; Lopes, C.; El-Amay, A.A.G.; Lindgren, M.; et al. Long Distance Enhancement of Nonlinear Optical Properties Using Low Concentration of Plasmonic Nanostructures in Dye Doped Monolithic Sol-Gel Materials. Adv. Funct. Mater. 2016, 26, 6005–6014. [Google Scholar] [CrossRef]
- Parola, S.; Julián-lópez, B.; Carlos, L.D.; Sanchez, C. Optical Properties of Hybrid Organic-Inorganic Materials and their Applications. Adv. Funct. Mater. 2016, 26, 6506–6544. [Google Scholar] [CrossRef]
- Andraud, C.; Fortrie, R.; Barsu, C.; Stéphan, O.; Chermette, H.; Baldeck, P.L. Excitonically Coupled Oligomers and Dendrimers for Two-Photon Absorption. In Photoresponsive Polymers II; Marder, S.R., Lee, K.-S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 149–203. ISBN 978-3-540-69454-0. [Google Scholar]
- Vivas, M.G.; De Boni, L.; Cooper, T.M.; Mendonca, C.R. Interpreting Strong Two-Photon Absorption of PE3 Platinum Acetylide Complex: Double Resonance and Excited State Absorption. ACS Photonics 2014, 1, 106–113. [Google Scholar] [CrossRef]
- Mettra, B.; Liao, Y.Y.; Gallavardin, T.; Armagnat, C.; Pitrat, D.; Baldeck, P.; Le Bahers, T.; Monnereau, C.; Andraud, C. A combined theoretical and experimental investigation on the influence of the bromine substitution pattern on the photophysics of conjugated organic chromophores. Phys. Chem. Chem. Phys. 2018, 20, 3768–3783. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.J.; Bolger, J.A.; Staromlynska, J. Linear and nonlinear optical properties of platinum-ethynyl. Appl. Phys. B 1998, 108, 5537–5541. [Google Scholar] [CrossRef]
- Glimsdal, E.; Carlsson, M.; Eliasson, B.; Minaev, B.; Lindgren, M. Excited states and two-photon absorption of some novel thiophenyl Pt(II)-ethynyl derivatives. J. Phys. Chem. A 2007, 111, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Glimsdal, E.; Carlsson, M.; Kindahl, T.; Lindgren, M.; Lopes, C.; Eliasson, B. Luminescence, Singlet Oxygen Production, and Optical Power Limiting of Some Diacetylide Platinum(II) Diphosphine Complexes. J. Phys. Chem. A 2010, 114, 3431–3442. [Google Scholar] [CrossRef]
- Glimsdal, E.; Dragland, I.; Carlsson, M.; Eliasson, B.; Melø, T.B.; Lindgren, M. Triplet Excited States of Some Thiophene and Triazole Substituted Platinum(II) Acetylide Chromophores. J. Phys. Chem. A 2009, 113, 3311–3320. [Google Scholar] [CrossRef]
- Enderlein, J. Simplex.m. Available online: https://www.joerg-enderlein.de/software (accessed on 1 November 2018).
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Vivas, M.G.; Piovesan, E.; Silva, D.L.; Cooper, T.M.; De Boni, L.; Mendonca, C.R. Broadband three-photon absorption spectra of platinum acetylide complexes. Opt. Mater. Express 2011, 1, 700–710. [Google Scholar] [CrossRef]
- Staromlynska, J.; McKay, T.J.; Wilson, P. Broadband optical limiting based on excited state absorption in Pt: ethynyl. J. Appl. Phys. 2000, 88, 1726–1732. [Google Scholar] [CrossRef]
- Bellier, Q.; Makarov, N.S.; Bouit, P.A.; Rigaut, S.; Kamada, K.; Feneyrou, P.; Berginc, G.; Maury, O.; Perry, J.W.; Andraud, C. Excited state absorption: A key phenomenon for the improvement of biphotonic based optical limiting at telecommunication wavelengths. Phys. Chem. Chem. Phys. 2012, 14, 15299–15307. [Google Scholar] [CrossRef] [PubMed]
- Lundén, H.; Glimsdal, E.; Lindgren, M.; Lopes, C. How to assess good candidate molecules for self-activated optical power limiting. Opt. Eng. 2018, 57, 030802. [Google Scholar] [CrossRef] [Green Version]
- Mckay, T.J.; Staromlynska, J.; Davy, J.R.; Bolger, J.A. Cross sections for excited-state absorption in a Pt: Ethynyl complex. J. Opt. Soc. Am. B 2001, 18, 358–362. [Google Scholar] [CrossRef]
- Hollins, R.C. Optical Limiters: Spatial, temporal, and spectral effects. Nonlinear Opt. 1999, 21, 49–61. [Google Scholar]
- Barsu, C. Les Ingénierie Moléculaire Pour L’optique Non-Linéaire: Dendrimères à Base de Fluorène Pour L’absorption à Deux Photons et Molécules Dipolaires Pour L’imagerie Biologique. Ph.D. Thesis, ENS de Lyon, Lyon, France, 2006. [Google Scholar]
- Liotta, A. Contrôle et études de matériaux hybrides et plasmoniques pour des applications optiques. Ph.D. Thesis, ENS de Lyon, Lyon, France, 2016. [Google Scholar]
- Lind, P.; Boström, D.; Carlsson, M.; Eriksson, A.; Glimsdal, E.; Lindgren, M.; Eliasson, B. Structural, Photophysical, and Nonlinear Absorption Properties of trans-Di-arylalkynyl Platinum(II) Complexes with Phenyl and Thiophenyl Groups. J. Phys. Chem. A 2007, 111, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Albota, M.A.; Xu, C.; Webb, W.W. Two-photon fluorescence excitation cross sections of biomolecular probes from 690 to 960 nm. Appl. Opt. 1998, 37, 7352–7356. [Google Scholar] [CrossRef] [Green Version]
- Vincent, D.; Cruickshank, J. Optical limiting with C60 and other fullerenes. Appl. Opt. 1997, 36, 7794–7798. [Google Scholar] [CrossRef]
- Hopen, D.K. Spectroscopic Characterization and Performance of Novel Triplet State Enhanced Molecules for Optical Power Limiting. Master’s Thesis, NTNU, Trondheim, Norway, 2018. [Google Scholar]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundén, H.; Pitrat, D.; Mulatier, J.-C.; Monnereau, C.; Minda, I.; Liotta, A.; Chábera, P.; Hopen, D.K.; Lopes, C.; Parola, S.; et al. An Optical Power Limiting and Ultrafast Photophysics Investigation of a Series of Multi-Branched Heavy Atom Substituted Fluorene Molecules. Inorganics 2019, 7, 126. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7100126
Lundén H, Pitrat D, Mulatier J-C, Monnereau C, Minda I, Liotta A, Chábera P, Hopen DK, Lopes C, Parola S, et al. An Optical Power Limiting and Ultrafast Photophysics Investigation of a Series of Multi-Branched Heavy Atom Substituted Fluorene Molecules. Inorganics. 2019; 7(10):126. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7100126
Chicago/Turabian StyleLundén, Hampus, Delphine Pitrat, Jean-Christophe Mulatier, Cyrille Monnereau, Iulia Minda, Adrien Liotta, Pavel Chábera, Didrik K. Hopen, Cesar Lopes, Stéphane Parola, and et al. 2019. "An Optical Power Limiting and Ultrafast Photophysics Investigation of a Series of Multi-Branched Heavy Atom Substituted Fluorene Molecules" Inorganics 7, no. 10: 126. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics7100126