Binding Properties of a Dinuclear Zinc(II) Salen-Type Molecular Tweezer with a Flexible Spacer in the Formation of Lewis Acid-Base Adducts with Diamines
Abstract
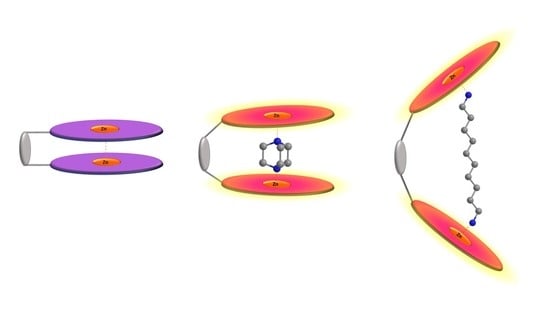
:1. Introduction
2. Results and Discussion
2.1. α,ω-Aliphatic Diamines
2.2. Rigid Diamines
2.3. Sensing Piperazine
3. Experimental Section
3.1. Materials and General Procedures
3.2. Physical Measurements
3.3. Calculation of Binding Constants and Limit of Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardouin–Lerouge, M.; Hudhomme, P.; Sallé, M. Molecular clips and tweezers hosting neutral guests. Chem. Soc. Rev. 2011, 40, 30–43. [Google Scholar] [CrossRef]
- Leblond, J.; Petitjean, A. Molecular Tweezers: Concepts and Applications. ChemPhysChem 2011, 12, 1043–1051. [Google Scholar] [CrossRef]
- Valderrey, V.; Aragay, G.; Ballester, P. Porphyrin tweezer receptors: Binding studies, conformational properties and applications. Co-Ord. Chem. Rev. 2014, 258-259, 137–156. [Google Scholar] [CrossRef]
- Klärner, F.-G.; Schrader, T. Aromatic Interactions by Molecular Tweezers and Clips in Chemical and Biological Systems. Acc. Chem. Res. 2012, 46, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zhou, W.; Li, D.; Chai, Y.; Xiang, Y.; Yuan, R. RNA-regulated molecular tweezers for sensitive fluorescent detection of microRNA from cancer cells. Biosens. Bioelectron. 2015, 71, 98–102. [Google Scholar] [CrossRef]
- Fokkens, M.; Schrader, T.; Klärner, F.-G. A Molecular Tweezer for Lysine and Arginine. J. Am. Chem. Soc. 2005, 127, 14415–14421. [Google Scholar] [CrossRef] [PubMed]
- Pérez, E.M.; Martín, N. Molecular tweezers for fullerenes. Pure Appl. Chem. 2010, 82, 523–533. [Google Scholar] [CrossRef]
- Mbarek, A.; Moussa, G.; Chain, J.L. Pharmaceutical Applications of Molecular Tweezers, Clefts and Clips. Molecules 2019, 24, 1803. [Google Scholar] [CrossRef] [Green Version]
- Hadrovic, I.; Rebmann, P.; Klärner, F.-G.; Bitan, G.; Schrader, T. Molecular Lysine Tweezers Counteract Aberrant Protein Aggregation. Front. Chem. 2019, 7, 657. [Google Scholar] [CrossRef]
- Schrader, T.; Bitan, G.; Klärner, F.-G. Molecular tweezers for lysine and arginine—Powerful inhibitors of pathologic protein aggregation. Chem. Commun. 2016, 52, 11318–11334. [Google Scholar] [CrossRef] [Green Version]
- Gianneschi, N.C.; Cho, S.-H.; Nguyen, S.; Mirkin, C.A. Reversibly Addressing an Allosteric Catalyst In Situ: Catalytic Molecular Tweezers. Angew. Chem. Int. Ed. 2004, 43, 5503–5507. [Google Scholar] [CrossRef]
- Doistau, B.; Benda, L.; Cantin, J.-L.; Cador, O.; Pointillart, F.; Wernsdorfer, W.; Chamoreau, L.-M.; Marvaud, V.; Hasenknopf, B.; Vives, G. Dual switchable molecular tweezers incorporating anisotropic MnIII–salphen complexes. Dalton Trans. 2020, 49, 8872–8882. [Google Scholar] [CrossRef]
- Doistau, B.; Tron, A.; Denisov, S.A.; Jonusauskas, G.; McClenaghan, N.D.; Gontard, G.; Marvaud, V.; Hasenknopf, B.; Vives, G. Terpy(Pt-salphen)2 Switchable Luminescent Molecular Tweezers. Chem. A Eur. J. 2014, 20, 15799–15807. [Google Scholar] [CrossRef] [Green Version]
- Doistau, B.; Benda, L.; Cantin, J.-L.; Chamoreau, L.-M.; Ruiz, E.; Marvaud, V.; Hasenknopf, B.; Vives, G. Six States Switching of Redox-Active Molecular Tweezers by Three Orthogonal Stimuli. J. Am. Chem. Soc. 2017, 139, 9213–9220. [Google Scholar] [CrossRef] [Green Version]
- Krykun, S.; Dekhtiarenko, M.; Canevet, D.; Carré, V.; Aubriet, F.; Levillain, E.; Allain, M.; Voitenko, Z.; Sallé, M.; Goeb, S. Metalla-Assembled Electron-Rich Tweezers: Redox-Controlled Guest Release Through Supramolecular Dimerization. Angew. Chem. Int. Ed. 2020, 59, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Han, Y.; Gao, Z.; Wang, F. Multicomponent Assembled Systems Based on Platinum(II) Terpyridine Complexes. Acc. Chem. Res. 2018, 51, 2719–2729. [Google Scholar] [CrossRef]
- Han, Y.; Tian, Y.; Li, Z.; Wang, F. Donor–acceptor-type supramolecular polymers on the basis of preorganized molecular tweezers/guest complexation. Chem. Soc. Rev. 2018, 47, 5165–5176. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tanasova, M.; Vasileiou, C.; Borhan, B. Fluorinated Porphyrin Tweezer: A Powerful Reporter of Absolute Configuration for erythro and threo Diols, Amino Alcohols, and Diamines. J. Am. Chem. Soc. 2008, 130, 1885–1893. [Google Scholar] [CrossRef]
- Huang, X.; Rickman, B.H.; Borhan, B.; Berova, N.; Nakanishi, K. Zinc Porphyrin Tweezer in Host−Guest Complexation: Determination of Absolute Configurations of Diamines, Amino Acids, and Amino Alcohols by Circular Dichroism. J. Am. Chem. Soc. 1998, 120, 6185–6186. [Google Scholar] [CrossRef]
- Borovkov, V.V.; Lintuluoto, J.M.; Hembury, G.A.; Sugiura, M.; Arakawa, R.; Inoue, Y. Supramolecular Chirogenesis in Zinc Porphyrins: Interaction with Bidentate Ligands, Formation of Tweezer Structures, and the Origin of Enhanced Optical Activity. J. Org. Chem. 2003, 68, 7176–7192. [Google Scholar] [CrossRef]
- Brahma, S.; Ikbal, S.A.; Rath, S.P. Synthesis, Structure, and Properties of a Series of Chiral Tweezer–Diamine Complexes Consisting of an Achiral Zinc(II) Bisporphyrin Host and Chiral Diamine Guest: Induction and Rationalization of Supramolecular Chirality. Inorg. Chem. 2014, 53, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Blom, M.; Norrehed, S.; Andersson, C.-H.; Huang, H.; Light, M.E.; Bergquist, J.; Grennberg, H.; Gogoll, A. Synthesis and Properties of Bis-Porphyrin Molecular Tweezers: Effects of Spacer Flexibility on Binding and Supramolecular Chirogenesis. Molecules 2015, 21, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norrehed, S.; Johansson, H.; Grennberg, H.; Gogoll, A. Improved Stereochemical Analysis of Conformationally Flexible Diamines by Binding to a Bisporphyrin Molecular Clip. Chem. A Eur. J. 2013, 19, 14631–14638. [Google Scholar] [CrossRef]
- Lubian, E.; Baldini, F.; Giannetti, A.; Trono, C.; Carofiglio, T. Solid-supported Zn(II) porphyrin tweezers as optical sensors for diamines. Chem. Commun. 2010, 46, 3678–3680. [Google Scholar] [CrossRef]
- Li, X.; Burrell, C.E.; Staples, R.J.; Borhan, B. Absolute Configuration for 1,n-Glycols: A Nonempirical Approach to Long-Range Stereochemical Determination. J. Am. Chem. Soc. 2012, 134, 9026–9029. [Google Scholar] [CrossRef]
- Li, X.; Borhan, B. Prompt Determination of Absolute Configuration for Epoxy Alcohols via Exciton Chirality Protocol. J. Am. Chem. Soc. 2008, 130, 16126–16127. [Google Scholar] [CrossRef]
- Crossley, M.J.; Hambley, T.W.; Mackay, L.G.; Try, A.C.; Walton, R. Porphyrin analogues of Tröger’s base: Large chiral cavities with a bimetallic binding site. J. Chem. Soc. Chem. Commun. 1995, 1077–1079. [Google Scholar] [CrossRef]
- Hayashi, T.; Nonoguchi, M.; Aya, T.; Ogoshi, H. Molecular recognition of α,ω-diamines by metalloporphyrin dimer. Tetrahedron Lett. 1997, 38, 1603–1606. [Google Scholar] [CrossRef]
- Carofiglio, T.; Lubian, E.; Menegazzo, I.; Saielli, G.; Varotto, A. Melamine-Bridged Bis(porphyrin-ZnII) Receptors: Molecular Recognition Properties. J. Org. Chem. 2009, 74, 9034–9043. [Google Scholar] [CrossRef]
- Norrehed, S.; Polavarapu, P.; Yang, W.; Gogoll, A.; Grennberg, H. Conformational restriction of flexible molecules in solution by a semirigid bis-porphyrin molecular tweezer. Tetrahedron 2013, 69, 7131–7138. [Google Scholar] [CrossRef] [Green Version]
- Consiglio, G.; Oliveri, I.P.; Failla, S.; Di Bella, S. On the Aggregation and Sensing Properties of Zinc(II) Schiff-Base Complexes of Salen-Type Ligands. Molecules 2019, 24, 2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leoni, L.; Dalla Cort, A. The Supramolecular Attitude of Metal–Salophen and Metal–Salen Complexes. Inorganics 2018, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.-Y.; Tang, J.; Zhang, J.-L. Introducing Metallosalens into Biological Studies: The Renaissance of Traditional Coordination Complexes. Eur. J. Inorg. Chem. 2017, 2017, 5085–5093. [Google Scholar] [CrossRef] [Green Version]
- Di Bella, S. Lewis acidic zinc(II) salen-type Schiff-base complexes: Sensing properties and responsive nanostructures. Dalton Trans. 2021, 50, 6050–6063. [Google Scholar] [CrossRef]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Purrello, R.; Di Bella, S. Supramolecular Aggregation/Deaggregation in Amphiphilic Dipolar Schiff-Base Zinc(II) Complexes. Inorg. Chem. 2010, 49, 5134–5142. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Di Bella, S.; Failla, S.; Malandrino, G. New molecular architectures by aggregation of tailored zinc(II) Schiff-base complexes. New J. Chem. 2011, 35, 2826–2831. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Di Bella, S. Sensitive Fluorescent Detection and Lewis Basicity of Aliphatic Amines. J. Phys. Chem. A 2011, 115, 14325–14330. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Di Bella, S. Highly sensitive fluorescent probe for detection of alkaloids. Tetrahedron 2011, 67, 9446–9449. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Di Bella, S. Lewis basicity of relevant monoanions in a non-protogenic organic solvent using a zinc(II) Schiff-base complex as a reference Lewis acid. Dalton Trans. 2017, 46, 11608–11614. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Malandrino, G.; Di Bella, S. Phase Transition and Vapochromism in Molecular Assemblies of a Polymorphic Zinc(II) Schiff-Base Complex. Inorg. Chem. 2014, 53, 9771–9777. [Google Scholar] [CrossRef]
- Mirabella, S.; Oliveri, I.P.; Ruffino, F.; Maccarrone, G.; Di Bella, S. Low-cost chemiresistive sensor for volatile amines based on a 2D network of a zinc(II) Schiff-base complex. Appl. Phys. Lett. 2016, 109, 143108. [Google Scholar] [CrossRef]
- Tang, J.; Cai, Y.-B.; Jing, J.; Zhang, J.-L. Unravelling the correlation between metal induced aggregation and cellular uptake/subcellular localization of Znsalen: An overlooked rule for design of luminescent metal probes. Chem. Sci. 2015, 6, 2389–2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Xie, D.; Yin, H.-Y.; Jing, J.; Zhang, J.-L. Cationic sulfonium functionalization renders Znsalens with high fluorescence, good water solubility and tunable cell-permeability. Org. Biomol. Chem. 2016, 14, 3360–3368. [Google Scholar] [CrossRef]
- Strianese, M.; Guarnieri, D.; Lamberti, M.; Landi, A.; Peluso, A.; Pellecchia, C. Fluorescent salen-type Zn(II) Complexes As Probes for Detecting Hydrogen Sulfide and Its Anion: Bioimaging Applications. Inorg. Chem. 2020, 59, 15977–15986. [Google Scholar] [CrossRef]
- Consiglio, G.; Oliveri, I.P.; Cacciola, S.; Maccarrone, G.; Failla, S.; Di Bella, S. Dinuclear zinc(II) salen-type Schiff-base complexes as molecular tweezers. Dalton Trans. 2020, 49, 5121–5133. [Google Scholar] [CrossRef]
- Munzi, G.; Failla, S.; Di Bella, S. Highly selective and sensitive colorimetric/fluorometric dual mode detection of relevant biogenic amines. Analyst 2021, 146, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, I.P.; Maccarrone, G.; Di Bella, S. A Lewis Basicity Scale in Dichloromethane for Amines and Common Nonprotogenic Solvents Using a Zinc(II) Schiff-Base Complex as Reference Lewis Acid. J. Org. Chem. 2011, 76, 8879–8884. [Google Scholar] [CrossRef]
- Forte, G.; Oliveri, I.P.; Consiglio, G.; Failla, S.; Di Bella, S. On the Lewis acidic character of bis(salicylaldiminato)zinc(II) Schiff-base complexes: A computational and experimental investigation on a series of compounds varying the bridging diimine. Dalton Trans. 2017, 46, 4571–4581. [Google Scholar] [CrossRef]
- She, N.; Moncelet, D.; Gilberg, L.; Lu, X.; Sindelar, V.; Briken, V.; Isaacs, L. Glycoluril-Derived Molecular Clips are Potent and Selective Receptors for Cationic Dyes in Water. Chem. A Eur. J. 2016, 22, 15270–15279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnett, C.A.; Witt, D.; Fettinger, J.C.; Isaacs, L. Acyclic Congener of Cucurbituril: Synthesis and Recognition Properties. J. Org. Chem. 2003, 68, 6184–6191. [Google Scholar] [CrossRef]
- Heilmann, M.; Tiefenbacher, K. A Modular Phosphorylated Glycoluril-Derived Molecular Tweezer for Potent Binding of Aliphatic Diamines. Chem. A Eur. J. 2019, 25, 12900–12904. [Google Scholar] [CrossRef]
- Solladié, N.; Aziat, F.; Bouatra, S.; Rein, R. Bis-porphyrin tweezers: Rigid or flexible linkers for better adjustment of the cavity to bidentate bases of various size. J. Porphyr. Phthalocyanines 2008, 12, 1250–1260. [Google Scholar] [CrossRef]
- Murphy, R.B.; Pham, D.-T.; White, J.M.; Lincoln, S.F.; Johnston, M.R. Molecular tweezers with a rotationally restricted linker and freely rotating porphyrin moieties. Org. Biomol. Chem. 2018, 16, 6206–6223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballester, P.; Costa, A.; Castilla, A.M.; Deyà, P.M.; Frontera, A.; Gomila, R.M.; Hunter, C.A.; Costa, A. DABCO-Directed Self-Assembly of Bisporphyrins (DABCO=1,4-Diazabicyclo[2.2.2]octane). Chem. A Eur. J. 2005, 11, 2196–2206. [Google Scholar] [CrossRef]
- Martin, R. Modes of action of anthelmintic drugs. Veter. J. 1997, 154, 11–34. [Google Scholar] [CrossRef]
- Feron, P.H.M.; Cousins, A.; Jiang, K.; Zhai, R.; Garcia, M. An update of the benchmark post-combustion CO2-capture technology. Fuel 2020, 273, 117776. [Google Scholar] [CrossRef]
- Mazari, S.; Ali, B.S.; Jan, B.M.; Saeed, I.M. Degradation study of piperazine, its blends and structural analogs for CO2 capture: A review. Int. J. Greenh. Gas Control. 2014, 31, 214–228. [Google Scholar] [CrossRef]
- Kumar, R.R.; Sahu, B.; Pathania, S.; Singh, P.K.; Akhtar, M.J.; Kumar, B. Piperazine, a Key Substructure for Antidepressants: Its Role in Developments and Structure-Activity Relationships. ChemMedChem 2021. [Google Scholar] [CrossRef]
- Katz, D.P.; Deruiter, J.; Bhattacharya, D.; Ahuja, M.; Bhattacharya, S.; Clark, C.; Suppiramaniam, V.; Dhanasekaran, M. Benzylpiperazine: “A messy drug”. Drug Alcohol Depend. 2016, 164, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Yiannias, J.A. Contact Dermatitis to Medications and Skin Products. Clin. Rev. Allergy Immunol. 2018, 56, 41–59. [Google Scholar] [CrossRef]
- Eide-Haugmo, I.; Brakstad, O.G.; Hoff, K.A.; Sørheim, K.R.; da Silva, E.F.; Svendsen, H.F. Environmental impact of amines. Energy Procedia 2009, 1, 1297–1304. [Google Scholar] [CrossRef] [Green Version]
- European Chemicals Bureau; Institute for Health and Consumer Protection E.U. Risk Assessment Report-Piperazine; Munn, S.J.; Allanou, R.; Aschberger, K.; Cosgrove, O.; Pakalin, S.; Paya-Perez, A.; Pellegrini, G.; Schwarz-Schulz, B.; et al. Office for Official Publications of the European Communities, Luxemburg. 2005. Available online: https://echa.europa.eu/documents/10162/35f9602c-cb84-448f-9383-250e1a5ad350 (accessed on 11 April 2021).
- El-Shabouri, S.R.; Mohamed, F.A.; Mohamed, A.M.I. A rapid spectrophotometric method for determination of piperazine. Talanta 1987, 34, 968–970. [Google Scholar] [CrossRef]
- Wahbi, A.-A.M.; Abounassif, M.A.; Gad-Kariem, E.A. Colorimetric determination of piperazine with p-benzoquinone. Talanta 1986, 33, 179–181. [Google Scholar] [CrossRef]
- Wahbi, A.-A.M.; Abounassif, M.; Kariem, E.A. Spectrophometric determination of piperazine with chloranil. Analyst 1984, 109, 1513–1514. [Google Scholar] [CrossRef]
- Muralikrishna, U.; Krishnamurthy, M.; Rao, N.S. Analytical uses of charge-transfer complexes: Determination of pure and dosage forms of piperazine. Analyst 1984, 109, 1277–1279. [Google Scholar] [CrossRef]
- Bu, X.; Pang, M.; Wang, B.; Zhang, Y.; Xie, K.; Zhao, X.; Wang, Y.; Guo, Y.; Liu, C.; Wang, R.; et al. Determination of Piperazine in Eggs Using Accelerated Solvent Extraction (ASE) and Solid Phase Extraction (SPE) with High-Performance Liquid Chromatography—Fluorescence Detection (HPLC-FLD) and Pre-Column Derivatization with Dansyl Chloride. Anal. Lett. 2019, 53, 53–71. [Google Scholar] [CrossRef]
- Hadi, M.; Ahmadvand, E.; Ehsani, A. Electroanalytical Sensing of Piperazine at Carbon Nanotubes/Nafion Composite-modified Glassy Carbon and Screen-printed Carbon Electrodes in Human Plasma. J. Anal. Chem. 2020, 75, 238–245. [Google Scholar] [CrossRef]
- Denis, C.M.; Baryla, N.E. Determination of piperazine in pharmaceutical drug substances using capillary electrophoresis with indirect UV detection. J. Chromatogr. A 2006, 1110, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Guanais Goncalves, C.; Dini, F.; Martinelli, E.; Catini, A.; Lundström, I.; Paolesse, R.; Di Natale, C. Detection of diverse potential threats in water with an array of optical sensors. Sens. Actuators B Chem. 2016, 236, 997–1004. [Google Scholar] [CrossRef]
- Lu, G.; Grossman, J.E.; Lambert, J.B. General but Discriminating Fluorescent Chemosensor for Aliphatic Amines. J. Org. Chem. 2006, 71, 1769–1776. [Google Scholar] [CrossRef]
- McGrier, P.L.; Solntsev, K.M.; Miao, S.; Tolbert, L.M.; Miranda, O.R.; Rotello, V.M.; Bunz, U.H.F. Hydroxycruciforms: Amine-Responsive Fluorophores. Chem. A Eur. J. 2008, 14, 4503–4510. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2010, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Bourson, J.; Pouget, J.; Valeur, B. Ion-responsive fluorescent compounds. 4. Effect of cation binding on the photophysical properties of a coumarin linked to monoaza- and diaza-crown ethers. J. Phys. Chem. 1993, 97, 4552–4557. [Google Scholar] [CrossRef]
- Currie, L.A. Detection and quantification limits: Origins and historical overview. Anal. Chim. Acta 1999, 391, 127–134. [Google Scholar] [CrossRef]
- Analytical Methods Committee, Recommendations for the definition, estimation and use of the detection limit. Analyst 1987, 112, 199–204. [CrossRef]










| Diamine | log K | Absorption λmax (nm) | Emission λmax (nm) |
|---|---|---|---|
| 1 | 582 | 625 | |
| PZ | 5.4 ± 0.1 | 580 | 618 |
| DABCO | 5.6 ± 0.2 | 580 | 614 |
| DPE | 4.0 ± 0.1 | 579 | 614 |
| BPY | 2.1 ± 0.2 (K1) 3.6 ± 0.2 (K2) | 580 | 611 |
| 1,2-Diaminoethane | - | 583 | 614 |
| 1,3-Diaminopropane | 2.9 ± 0.1 | 580 | 614 |
| 1,4-Diaminobutane b | 4.3 ± 0.1 | 580 | 618 |
| 1,5-Diaminopentane b | 5.0 ± 0.2 | 580 | 618 |
| 1,6-Diaminohexane | 5.1 ± 0.1 | 580 | 614 |
| 1,8-Diaminooctane | 6.4 ± 0.1 | 587 | 616 |
| 1,10-Diaminodecane | 6.2 ± 0.2 | 580 | 614 |
| 1,12-Diaminododecane | 5.9 ± 0.2 | 580 | 614 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munzi, G.; Consiglio, G.; Failla, S.; Di Bella, S. Binding Properties of a Dinuclear Zinc(II) Salen-Type Molecular Tweezer with a Flexible Spacer in the Formation of Lewis Acid-Base Adducts with Diamines. Inorganics 2021, 9, 49. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9060049
Munzi G, Consiglio G, Failla S, Di Bella S. Binding Properties of a Dinuclear Zinc(II) Salen-Type Molecular Tweezer with a Flexible Spacer in the Formation of Lewis Acid-Base Adducts with Diamines. Inorganics. 2021; 9(6):49. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9060049
Chicago/Turabian StyleMunzi, Gabriella, Giuseppe Consiglio, Salvatore Failla, and Santo Di Bella. 2021. "Binding Properties of a Dinuclear Zinc(II) Salen-Type Molecular Tweezer with a Flexible Spacer in the Formation of Lewis Acid-Base Adducts with Diamines" Inorganics 9, no. 6: 49. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9060049