Synthesis and Characterization of Super Bulky β-Diketiminato Group 1 Metal Complexes
Abstract
:1. Introduction
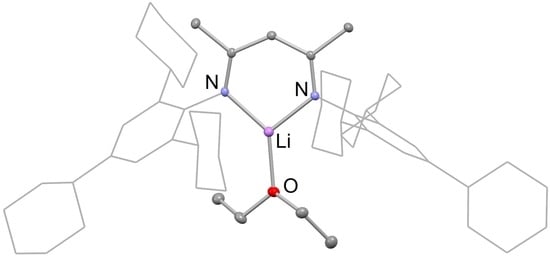
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Syntehsis of TCHP/DipNacnacH 2 and Data for TCHPNacacH 3
3.3. Syntehsis of Complexes 4–7
3.4. Crystallographic Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourget-Merle, L.; Lappert, M.F.; Severn, J.R. The Chemistry of β-Diketiminatometal Complexes. Chem. Rev. 2002, 102, 3031–3066. [Google Scholar] [CrossRef] [PubMed]
- Sarish, S.P.; Nembenna, S.; Nagendran, S.; Roesky, H.W. Chemistry of Soluble β-Diketiminatoalkaline-Earth Metal Complexes with M−X Bonds (M = Mg, Ca, Sr; X = OH, Halides, H). Acc. Chem. Res. 2011, 44, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Driess, M. Lessons from Isolable Nickel(I) Precursor Complexes for Small Molecule Activation. Acc. Chem. Res. 2012, 45, 276–287. [Google Scholar] [CrossRef]
- Tsai, Y. The chemistry of univalent metal β-diketiminates. Coord. Chem. Rev. 2012, 256, 722–758. [Google Scholar] [CrossRef]
- Zhu, D.; Budzelaar, P.H.M. N-Aryl β-diiminate complexes of the platinum metals. Dalton Trans. 2013, 42, 11343–11354. [Google Scholar] [CrossRef]
- Chen, C.; Bellows, S.M.; Holland, P.L. Tuning steric and electronic effects in transition-metal β-diketiminate complexes. Dalton Trans. 2015, 44, 16654–16670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohloch, S.; Kriegel, B.M.; Bergman, R.G.; Arnold, J. Group 5 chemistry supported by β-diketiminate ligands. Dalton Trans. 2016, 45, 15725–15745. [Google Scholar] [CrossRef]
- Camp, C.; Arnold, J. On the non-innocence of “Nacnacs”: Ligand-based reactivity in β-diketiminate supported coordination compounds. Dalton Trans. 2016, 45, 14462–14498. [Google Scholar] [CrossRef]
- Green, S.P.; Jones, C.; Stasch, A. Stable Magnesium(I) Compounds with Mg-Mg Bonds. Science 2007, 318, 1754–1757. [Google Scholar] [CrossRef]
- Stasch, A.; Jones, C. Stable dimeric magnesium(i) compounds: From chemical landmarks to versatile reagents. Dalton Trans. 2011, 40, 5659–5672. [Google Scholar] [CrossRef]
- Jones, C. Dimeric magnesium(I) β-diketiminates: A new class of quasi-universal reducing agent. Nat. Rev. Chem. 2017, 1, 0059. [Google Scholar] [CrossRef]
- Bonyhady, S.; Jones, C.; Nembenna, S.; Stasch, A.; Edwards, A.; McIntyre, G. β-Diketiminate-Stabilized Magnesium(I) Dimers and Magnesium(II) Hydride Complexes: Synthesis, Characterization, Adduct Formation, and Reactivity Studies. Chem. Eur. J. 2010, 16, 938–955. [Google Scholar] [CrossRef]
- Kenward, A.L.; Ross, J.A.; Piers, W.E.; Parvez, M. Metalation-Resistant β-Diketiminato Ligands for Thermally Robust Organoscandium Complexes. Organometallics 2009, 28, 3625–3628. [Google Scholar] [CrossRef]
- Cowley, R.E.; Holland, P.L. Ligand Effects on Hydrogen Atom Transfer from Hydrocarbons to Three-Coordinate Iron Imides. Inorg. Chem. 2012, 51, 8352–8361. [Google Scholar] [CrossRef]
- Arrowsmith, M.; Maitland, B.; Kociok-Köhn, G.; Stasch, A.; Jones, C.; Hill, M.S. Mononuclear Three-Coordinate Magnesium Complexes of a Highly Sterically Encumbered β-Diketiminate Ligand. Inorg. Chem. 2014, 53, 10543–10552. [Google Scholar] [CrossRef] [Green Version]
- Gentner, T.X.; Rösch, B.; Ballmann, G.; Langer, J.; Elsen, H.; Harder, S. Low Valent Magnesium Chemistry with a Super Bulky β-Diketiminate Ligand. Angew. Chem. Int. Ed. 2019, 58, 607–611. [Google Scholar] [CrossRef]
- Yuvaraj, K.; Douair, I.; Jones, D.D.L.; Maron, L.; Jones, C. Sterically controlled reductive oligomerisations of CO by activated magnesium(i) compounds: Deltate vs. ethenediolate formation. Chem. Sci. 2020, 11, 3516–3522. [Google Scholar] [CrossRef] [Green Version]
- Boutland, A.J.; Dange, D.; Stasch, A.; Maron, L.; Jones, C. Two-Coordinate Magnesium(I) Dimers Stabilized by Super Bulky Amido Ligands. Angew. Chem. Int. Ed. 2016, 55, 9239–9243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuvaraj, K.; Douair, I.; Paparo, A.; Maron, L.; Jones, C. Reductive Trimerization of CO to the Deltate Dianion Using Activated Magnesium(I) Compounds. J. Am. Chem. Soc. 2019, 141, 8764–8768. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, K.; Douair, I.; Maron, L.; Jones, C. Activation of Ethylene by N-Heterocyclic Carbene Coordinated Magnesium(I) Compounds. Chem. Eur. J. 2020, 26, 14665–14670. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.D.L.; Douair, I.; Maron, L.; Jones, C. Photochemically Activated Dimagnesium(I) Compounds: Reagents for the Reduction and Selective C−H Bond Activation of Inert Arenes. Angew. Chem. Int. Ed. 2021, 60, 7087–7092. [Google Scholar] [CrossRef]
- Rösch, B.; Gentner, T.X.; Eyselein, J.; Langer, J.; Elsen, H.; Harder, S. Strongly reducing magnesium(0) complexes. Nature 2021, 592, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Jones, C. Highly reactive form of magnesium stabilized by bulky ligands. Nature 2021, 592, 687–688. [Google Scholar] [CrossRef] [PubMed]
- Rösch, B.; Gentner, T.X.; Langer, J.; Färber, C.; Eyselein, J.; Zhao, L.; Frenking, G.; Harder, S. Dinitrogen complexation and reduction at low-valent calcium. Science 2021, 371, 1125–1128. [Google Scholar] [CrossRef]
- Weerawardhana, E.A.; Pena, A.; Zeller, M.; Lee, W.-T. Synthesis and characterization of iron and cobalt complexes with an asymmetric N-alkyl,N′-aryl-β-diketiminate ligand. Inorg. Chim. Acta 2017, 460, 29–34. [Google Scholar] [CrossRef]
- Stender, M.; Wright, R.J.; Eichler, B.E.; Prust, J.; Olmstead, M.M.; Roesky, H.W.; Power, P.P. The synthesis and structure of lithium derivatives of the sterically encumbered β-diketiminate ligand [{(2,6-Pri2H3C6)N(CH3)C}2CH]−, and a modified synthesis of the aminoimine precursor. J. Chem. Soc. Dalton Trans. 2001, 3465–3469. [Google Scholar] [CrossRef]
- Clegg, W.; Cope, E.K.; Edwards, A.J.; Mair, F.S. Structural Characterization of [(2,6-Pri2C6H3)NC(Me)C(H)C(Me)N(2,6-Pri2C6H3)K·PhCH3]∞: A Heavy Alkali Metal Diazapentadienyl Complex. Inorg. Chem. 1998, 37, 2317–2319. [Google Scholar] [CrossRef]
- Pyykö, P.; Atsumi, M. Molecular Single-Bond Covalent Radii for Elements 1–118. Chem. Eur. J. 2009, 15, 186–197. [Google Scholar] [CrossRef]
- Mantina, M.; Chamberlain, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals Radii for the Whole Main Group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dove, A.P.; Gibson, V.C.; Marshall, E.L.; White, A.J.; Williams, D.J. Magnesium and zinc complexes of a potentially tridentate β-diketiminate ligand. Dalton Trans. 2004, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Savka, R.; Plenio, H. Metal Complexes of Very Bulky N,N′-Diarylimidazolylidene N-Heterocyclic Carbene (NHC) Ligands with 2,4,6-Cycloalkyl Substituents. Eur. J. Inorg. Chem. 2014, 2014, 6246–6253. [Google Scholar] [CrossRef]
- Kundu, S.; Sinhababu, S.; Siddiqui, S.S.; Luebben, A.V.; Dittrich, B.; Yang, T.; Frenking, G.; Roesky, H.W. Comparison of Two Phosphinidenes Binding to Silicon(IV)dichloride as well as to Silylene. J. Am. Chem. Soc. 2018, 140, 9409–9412. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELX-16. University of Göttingen, 2016. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, D.D.L.; Watts, S.; Jones, C. Synthesis and Characterization of Super Bulky β-Diketiminato Group 1 Metal Complexes. Inorganics 2021, 9, 72. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9090072
Jones DDL, Watts S, Jones C. Synthesis and Characterization of Super Bulky β-Diketiminato Group 1 Metal Complexes. Inorganics. 2021; 9(9):72. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9090072
Chicago/Turabian StyleJones, Dafydd D. L., Samuel Watts, and Cameron Jones. 2021. "Synthesis and Characterization of Super Bulky β-Diketiminato Group 1 Metal Complexes" Inorganics 9, no. 9: 72. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9090072