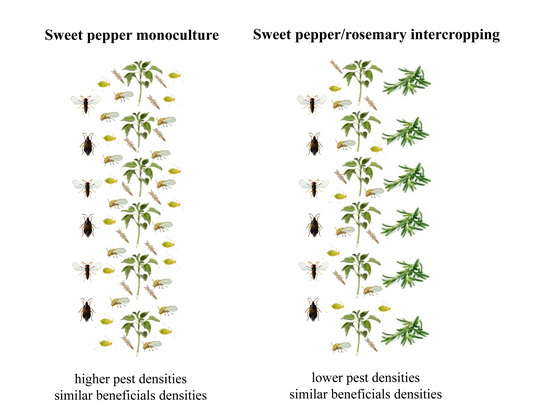
Intercropping Rosemary (Rosmarinus officinalis) with Sweet Pepper (Capsicum annum) Reduces Major Pest Population Densities without Impacting Natural Enemy Populations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Setup
2.2. Natural Enemies Release
2.3. Sampling of Pests and Natural Enemies on Sweet Pepper
2.4. Statistical Analysis
3. Results
3.1. Effect of Rosemary Intercropping on Population Dynamics of Pest Species
3.2. Effects of Rosemary Intercropping on Population Dynamics of Natural Enemies
3.3. Effect of Natural Enemy Release on Pest Densities and Pest-Natural Enemy Regressions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brewer, M.J.; Goodell, P.B. Approaches and incentives to implement integrated pest management that addresses regional and environmental issues. Annu. Rev. Entomol. 2011, 57, 41–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodeur, J.; Abram, P.K.; Heimpel, G.E.; Messing, R.H. Trends in biological control: Public interest, international networking and research direction. Biocontrol 2018, 63, 11–26. [Google Scholar] [CrossRef]
- Hassanali, A.; Herren, H.; Khan, Z.R.; Pickett, J.A.; Woodcock, C.M. Integrated pest management: The push-pull approach for controlling insect pests and weeds of cereals, and its potential for other agricultural systems including animal husbandry. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Shrivastava, G.; Rogers, M.; Wszelaki, A.; Panthee, D.R.; Chen, F. Plant volatiles-based insect pest management in organic farming. Crit. Rev. Plant Sci. 2010, 29, 123–133. [Google Scholar] [CrossRef]
- Beck, J.J.; Torto, B.; Vannette, R.L. Eavesdropping on plant-insect-microbe chemical communications in agricultural ecology: A virtual issue on semiochemicals. J. Agric. Food Chem. 2017, 65, 5101–5103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhnle, A.; Muller, C. Relevance of visual and olfactory cues for host location in the mustard leaf beetle Phaedon cochleariae. Physiol. Entomol. 2011, 36, 68–76. [Google Scholar] [CrossRef]
- Wynde, F.J.H.; Port, G.R. The use of olfactory and visual cues in host choice by the capsid bugs Lygus rugulipennis Poppius and Liocoris tripustulatus Fabricius. PLoS ONE 2012, 7, e46448. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Regnault-Roger, C. The potential of botanical essential oils for insect pest control. Integr. Pest Manag. Rev. 1997, 2, 25–34. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Finch, S.; Collier, R.H. The influence of host and non-host companion plants on the behaviour of pest insects in field crops. Entomol. Exp. Appl. 2011, 142, 87–96. [Google Scholar] [CrossRef]
- Finch, S.; Billiald, H.; Collier, R.H. Companion planting—Do aromatic plants disrupt host-plant finding by the cabbage root fly and the onion fly more effectively than non-aromatic plants? Entomol. Exp. Appl. 2003, 109, 183–195. [Google Scholar] [CrossRef]
- Finch, S.; Collier, R.H. Host-plant selection by insects—A theory based on ‘appropriate/inappropriate landings’ by pest insects of cruciferous plants. Entomol. Exp. Appl. 2000, 96, 91–102. [Google Scholar] [CrossRef]
- Tang, G.B.; Song, B.Z.; Zhao, L.L.; Sang, X.S.; Wan, H.H.; Zhang, J.; Yao, Y.C. Repellent and attractive effects of herbs on insects in pear orchards intercropped with aromatic plants. Agrofor. Syst. 2013, 87, 273–285. [Google Scholar] [CrossRef]
- Song, B.; Zhang, J.; Wiggins, N.L.; Yao, Y.; Tang, G.; Sang, X. Intercropping with aromatic plants decreases herbivore abundance, species richness, and shifts arthropod community trophic structure. Environ. Entomol. 2012, 41, 872–879. [Google Scholar] [CrossRef]
- Song, B.Z.; Wu, H.Y.; Kong, Y.; Zhang, J.; Du, Y.L.; Hu, J.H.; Yao, Y.C. Effects of intercropping with aromatic plants on the diversity and structure of an arthropod community in a pear orchard. Biocontrol 2010, 55, 741–751. [Google Scholar] [CrossRef]
- Carvalho, M.G.; Bortolotto, O.C.; Ventura, M.U. Aromatic plants affect the selection of host tomato plants by Bemisia tabaci biotype B. Entomol. Exp. Appl. 2017, 162, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Hatt, S.; Xu, Q.; Francis, F.; Osawa, N. Aromatic plants of East Asia to enhance natural enemies towards biological control of insect pests. Entomol. Gen. 2019, 38, 275–315. [Google Scholar] [CrossRef]
- Batista, M.C.; Fonseca, M.C.M.; Teodoro, A.V.; Martins, E.F.; Pallini, A.; Venzon, M. Basil (Ocimum basilicum L.) attracts and benefits the green lacewing Ceraeochrysa cubana Hagen. Biol. Control 2017, 110, 98–106. [Google Scholar] [CrossRef]
- Togni, P.H.B.; Venzon, M.; Muniz, C.A.; Martins, E.F.; Pallini, A.; Sujii, E.R. Mechanisms underlying the innate attraction of an aphidophagous coccinellid to coriander plants: Implications for conservation biological control. Biol. Control 2016, 92, 77–84. [Google Scholar] [CrossRef]
- Cullen, R.; Warner, K.D.; Jonsson, M.; Wratten, S.D. Economics and adoption of conservation biological control. Biol. Control 2008, 45, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Andow, D.A. Vegetational diversity and arthropod population response. Annu. Rev. Entomol. 1991, 36, 561–586. [Google Scholar] [CrossRef]
- Lopes, T.C.M.; Hatt, S.; Xu, Q.; Chen, J.; Francis, F. Wheat (Triticum aestivum L.)-based intercropping systems for biological pest control. Pest Manag. Sci. 2016, 72, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, L.M.; Saldanha, A.V.; Souza, D.R.; Viana, R.S.; Bordin, B.C.; Antonio, A.C. Intercropping hampers the nocturnal biological control of aphids. Ann. Appl. Biol. 2018, 172, 148–159. [Google Scholar] [CrossRef]
- Xie, J.; VanAlstyne, P.; Uhlir, A.; Yang, X. A review on rosemary as a natural antioxidation solution. Eur. J. Lipid Sci. Technol. 2017, 119, 1600439. [Google Scholar] [CrossRef]
- Ngo, S.N.T.; Williams, D.B.; Head, R.J. Rosemary and cancer prevention: Preclinical perspectives. Crit. Rev. Food Sci. Nutr. 2011, 51, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Omri, A.E.; Han, J.; Yamada, P.; Kawada, K.; Abdrabbah, M.B.; Isoda, H. Rosmarinus officinalis polyphenols activate cholinergic activities in PC12 cells through phosphorylation of ERK1/2. J. Ethnopharmacol. 2010, 131, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Dganit, S.; Nadav, N.; Alona, S.; David, C.; Nativ, D.; Murad, G.; Xiao-Wei, W. Whitefly attraction to rosemary (Rosmarinus officinialis L.) is associated with volatile composition and quantity. PLoS ONE 2017, 12, e0177483. [Google Scholar] [CrossRef]
- Katerinopoulos, H.E.; Pafona, G.; Afratis, A.; Stratigakis, N.; Roditakis, N. Composition and insect attracting activity of the essential oil of Rosmarinus officinalis. J. Chem. Ecol. 2005, 31, 111–122. [Google Scholar] [CrossRef]
- Koschier, E.H.; Sedy, K.A. Labiate essential oils affecting host selection and acceptance of Thrips tabaci lindeman. Crop Prot. 2003, 22, 929–934. [Google Scholar] [CrossRef]
- Sadeh, D.; Nitzan, N.; Shachter, A.; Ghanim, M.; Dudai, N. Rosemary-whitefly interaction: A continuum of repellency and volatile combinations. J. Econ. Entomol. 2019, 112, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, Z.; Gao, Y.; Bian, L.; Sun, X.; Chen, Z. Volatiles from non-host aromatic plants repel tea green leafhopper Empoasca vitis. Entomol. Exp. Appl. 2014, 153, 156–169. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Sun, X.-L.; Xin, Z.-J.; Luo, Z.-X.; Gao, Y.; Bian, L.; Chen, Z.-M. Identification and field evaluation of non-host volatiles disturbing host location by the tea geometrid, Ectropis obliqua. J. Chem. Ecol. 2013, 39, 1284–1296. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [Green Version]
- Pyke, B.; Rice, M.; Sabine, B.; Zalucki, M.P. The push-pull strategy—Behavioural control of Heliothis. Aust. Cotton Grow. 1987, 9, 7–9. [Google Scholar]
- Miresmailli, S.; Bradbury, R.; Isman, M.B. Comparative toxicity of Rosmarinus officinalis L. essential oil and blends of its major constituents against Tetranychus urticae Koch (Acari: Tetranychidae) on two different host plants. Pest Manag. Sci. 2006, 62, 366–371. [Google Scholar] [CrossRef]
- Hori, M. Repellency of rosemary oil against Myzus persicae in a laboratory and in a screenhouse. J. Chem. Ecol. 1998, 24, 1425–1432. [Google Scholar] [CrossRef]
- Hori, M.; Komatsu, H. Repellency of rosemary oil and its components against onion aphid, Neotoxoptera formosana (Takahashi) (Homoptera: Aphididae). Appl. Entomol. Zool. 1997, 32, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Dardouri, T.; Gomez, L.; Schoeny, A.; Costagliola, G.; Gautier, H. Behavioural response of green peach aphid Myzus persicae (Sulzer) to volatiles from different rosemary (Rosmarinus officinalis L.) clones. Agric. For. Entomol. 2019, 21, 336–345. [Google Scholar] [CrossRef]
- Bennison, J.; Maulden, K.; Dewhirst, S.; Pow, E.; Slatter, P.; Wadhams, L. Towards the development of a push-pull strategy for improving biological control of western flower thrips on chrysanthemum. In Proceedings of the Seventh International Symposium on Thysanoptera: Thrips, Plants, Tospoviruses: The Millenial Review, Reggio, Calabria, Italy, 2–7 July 2001; pp. 199–206. [Google Scholar]
- Li, X.; Zhang, Z.; Hafeez, M.; Huang, J.; Zhang, J.; Wang, L.; Lu, Y. Rosmarinus officinialis L. (Lamiales: Lamiaceae), a promising repellent plant for thrips management. J. Econ. Entomol. 2020. [Google Scholar] [CrossRef]
- Ben Issa, R.; Gautier, H.; Costagliola, G.; Gomez, L. Which companion plants affect the performance of green peach aphid on host plants? Testing of 12 candidate plants under laboratory conditions. Entomol. Exp. Appl. 2016, 160, 164–178. [Google Scholar] [CrossRef]
- Ben Issa, R.; Gautier, H.; Gomez, L. Influence of neighbouring companion plants on the performance of aphid populations on sweet pepper plants under greenhouse conditions. Agric. For. Entomol. 2017, 19, 181–191. [Google Scholar] [CrossRef]
- Weintraub, P.G. Integrated control of pests in tropical and subtropical sweet pepper production. Pest Manag. Sci. 2007, 63, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Bouagga, S.; Urbaneja, A.; Pérez-Hedo, M. Combined use of predatory mirids with Amblyseius swirskii (Acari: Phytoseiidae) to enhance pest management in sweet pepper. J. Econ. Entomol. 2018, 111, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Bouagga, S.; Urbaneja, A.; Pérez-Hedo, M. Comparative biocontrol potential of three predatory mirids when preying on sweet pepper key pests. Biol. Control 2018, 121, 168–174. [Google Scholar] [CrossRef]
- Reitz, S.R.; Gao, Y.L.; Kirk, W.D.J.; Hoddle, M.S.; Leiss, K.A.; Funderburk, J.E. Invasion biology, ecology, and management of western flower thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Arnó, J.; Roig, J.; Riudavets, J. Evaluation of Orius majusculus and O. laevigatus as predators of Bemisa tabaci and estimation of their prey preference. Biol. Control 2008, 44, 1–6. [Google Scholar] [CrossRef]
- Alvarado, P.; Baltà, O.; Alomar, O. Efficiency of four Heteroptera as predators of Aphis gossypii and Macrosiphum euphorbiae (Hom.: Aphididae). Entomophaga 1997, 42, 215–226. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Xie, W.; Wu, Q.; Wang, S. The suitability of biotypes Q and B of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) at different nymphal instars as hosts for Encarsia formosa Gahan (Hymenoptera: Aphelinidae). PeerJ 2016, 4, e1863. [Google Scholar] [CrossRef] [Green Version]
- Hoddle, M.S.; Van Driesche, R.G.; Sanderson, J.P. Biology and use of the whitefly parasitoid Encarsia formosa. Annu. Rev. Entomol. 1998, 43, 645–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IBM Corporation. SPSS for Windows, Version 22.0; IBM Corporation: Chicago, FL, USA, 2013. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Version 4.0.3; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Uvah, I.I.I.; Coaker, T.H. Effect of mixed cropping on some insect pests of carrots and onions. Entomol. Exp. Appl. 1984, 36, 159–167. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Schlyter, F. Redundancy, synergism, and active inhibitory range of non-host volatiles in reducing pheromone attraction in European spruce bark beetle Ips typographus. Oikos 2003, 101, 299–310. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Bueno, V.H.P.; Castañé, C. Olfactory response towards its prey Frankliniella occidentalis of wild and laboratory-reared Orius insidiosus and Orius laevigatus. J. Appl. Entomol. 2011, 135, 177–183. [Google Scholar] [CrossRef]
- Mochizuki, M.; Yano, E. Olfactory response of the anthocorid predatory bug Orius sauteri to thrips-infested eggplants. Entomol. Exp. Appl. 2007, 123, 57–62. [Google Scholar] [CrossRef]
- Inbar, M.; Gerling, D. Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Annu. Rev. Entomol. 2007, 53, 431–448. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.-G.; Ma, B.; Cheng, J.-A. Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper Nilaparvata lugens. J. Chem. Ecol. 2005, 31, 2357–2372. [Google Scholar] [CrossRef]
- Lin, Q.-C.; Chen, H.; Babendreier, D.; Zhang, J.-P.; Zhang, F.; Dai, X.-Y.; Sun, Z.-W.; Shi, Z.-P.; Dong, X.-L.; Wu, G.-A.; et al. Improved control of Frankliniella occidentalis on greenhouse pepper through the integration of Orius sauteri and neonicotinoid insecticides. J. Pest Sci. 2020. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, X.; Tan, X.; Desneux, N.; Zappala, L.; Zhang, F.; Wang, S. Using Calendula officinalis as a floral resource to enhance aphid and thrips suppression by the flower bug Orius sauteri (Hemiptera: Anthocoridae). Pest Manag. Sci. 2017, 73, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.-L.; Wu, Y.-Q.; Duan, Y.; Gao, X.-G. Control efficiencies of releasing Orius sauteri (Heteroptera:Anthocoridae) on some pests in greenhouse pepper. Chin. J. Biol. Control 2011, 27, 414–417. [Google Scholar] [CrossRef]
- Yin, Z.; Li, J.; Dong, M.; Hou, Z.; Sun, B.; Guo, X. Research on predation capacity and preference of Orius sauteri against western flower thrips (Frankliniella occidentalis), two-spotted spider mite (Tetranychus urticae) and peach aphid (Myzus persicae). China Plant Prot. 2017, 37, 17–19. [Google Scholar]
- Li, S.; Lao, S.-B.; Wang, S.; Guo, X.-J.; Zhang, F. Control effect of Orius sauteri collaborated with Encarsia formosa on Bemisia tabaci in the greenhouse. J. Environ. Entomol. 2014, 36, 978–982. [Google Scholar]
- Quicke, D. Parasitic Wasps; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Ju, Q.; Ouyang, F.; Gu, S.; Qiao, F.; Yang, Q.; Qu, M.; Ge, F. Strip intercropping peanut with maize for peanut aphid biological control and yield enhancement. Agric. Ecosyst. Environ. 2019, 286, 106682. [Google Scholar] [CrossRef]
- Xu, Q.; Hatt, S.; Lopes, T.; Zhang, Y.; Bodson, B.; Chen, J.; Francis, F. A push–pull strategy to control aphids combines intercropping with semiochemical releases. J. Pest Sci. 2018, 91, 93–103. [Google Scholar] [CrossRef]
- Hatt, S.; Lopes, T.; Boeraeve, F.; Chen, J.; Francis, F. Pest regulation and support of natural enemies in agriculture: Experimental evidence of within field wildflower strips. Ecol. Eng. 2017, 98, 240–245. [Google Scholar] [CrossRef]
- Ouyang, F.; Su, W.; Zhang, Y.; Liu, X.; Su, J.; Zhang, Q.; Men, X.; Ju, Q.; Ge, F. Ecological control service of the predatory natural enemy and its maintaining mechanism in rotation-intercropping ecosystem via wheat-maize-cotton. Agric. Ecosyst. Environ. 2020, 301, 107024. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-w.; Lu, X.-x.; Zhang, Z.-j.; Huang, J.; Zhang, J.-m.; Wang, L.-k.; Hafeez, M.; Fernández-Grandon, G.M.; Lu, Y.-b. Intercropping Rosemary (Rosmarinus officinalis) with Sweet Pepper (Capsicum annum) Reduces Major Pest Population Densities without Impacting Natural Enemy Populations. Insects 2021, 12, 74. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12010074
Li X-w, Lu X-x, Zhang Z-j, Huang J, Zhang J-m, Wang L-k, Hafeez M, Fernández-Grandon GM, Lu Y-b. Intercropping Rosemary (Rosmarinus officinalis) with Sweet Pepper (Capsicum annum) Reduces Major Pest Population Densities without Impacting Natural Enemy Populations. Insects. 2021; 12(1):74. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12010074
Chicago/Turabian StyleLi, Xiao-wei, Xin-xin Lu, Zhi-jun Zhang, Jun Huang, Jin-ming Zhang, Li-kun Wang, Muhammad Hafeez, G. Mandela Fernández-Grandon, and Yao-bin Lu. 2021. "Intercropping Rosemary (Rosmarinus officinalis) with Sweet Pepper (Capsicum annum) Reduces Major Pest Population Densities without Impacting Natural Enemy Populations" Insects 12, no. 1: 74. https://0-doi-org.brum.beds.ac.uk/10.3390/insects12010074