Assessing the Impact of Insecticide Resistance on Vector Competence: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Eligibility Criteria
2.2. Data Extraction and Synthesis
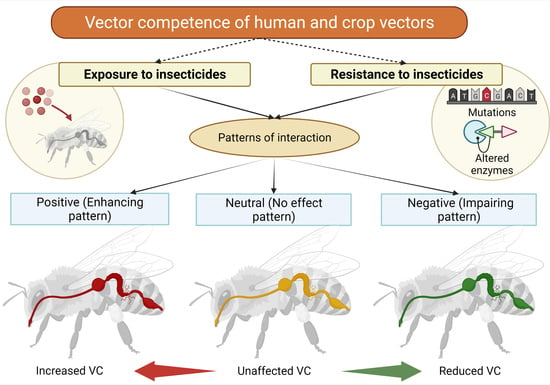
3. Results
3.1. Field Versus Laboratory Studies
3.2. Effects of Insecticide Exposure on Pathogen Transmission
3.3. Impacts of Insecticide Resistance on Pest Vectors in Crops
| Species | Pathogen | Insecticide Exposure | Metabolic Resistance | Target Site Modifications | Phenotypic Resistance | Type of Association | Location | Additional Treatments | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Anopheles gambiae | Plasmodium berghei | DDT | GST | DDT | Positive | Lab | [57] | ||
| An.gambiae s.s. | Plasmodium falciparum | L1014S | Deltamethrin | Positive | Field | [37] | |||
| Plasmodium falciparum | DDT | L1014F | DDT | Positive | Field | [38] | |||
| Plasmodium falciparum | L1014F, G119S | OP, CAR, and PYR-DDT | Negative 1 | Lab | [51] | ||||
| Plasmodium falciparum | L1014F, G119S | OP, CAR, and PYR-DDT | Positive 2 | Lab | [51] | ||||
| Metarhizium 3 anisopliae | L1014F | PYR | Positive | Lab | [56] | ||||
| Beauveria bassiana 3 | L1014F | PYR | Positive | Lab | [56] | ||||
| Plasmodium falciparum | Deltamethrin | L1014S | Negative | Field | [44] | ||||
| Plasmodium berghei | Permethrin | Negative | Lab | Larval competition | [71] | ||||
| An.s gambiae s. l. | Plasmodium falciparum | α-Cypermethrin, Deltamethrin, Permethrin | N1575Y, I1527T, L1014F, G119S | PYR | Neutral | Field | [40] | ||
| Plasmodium sp. | L1014F, G119S | Neutral | Field | [41] | |||||
| Plasmodium falciparum | L1014F, L1014S | Positive | Lab | [52] | |||||
| An. funestus | Plasmodium falciparum | L119F-GSTe2 | Negative 1 | Lab | [50] | ||||
| Plasmodium falciparum | L119F-GSTe2 | Positive 2 | Lab | [50] | |||||
| Plasmodium sp. | L119F-GSTe2 | Neutral | Field | [39] | |||||
| Plasmodium sp. | L119F-GSTe2 | Positive | Field | [39] | |||||
| Plasmodium sp. | A296S (GABA) | Negative | Field | [39] | |||||
| Culex gelidus | Japanese Encephalitis Virus | Deltamethrin, Malathion | Neutral | Field | [45] | ||||
| Cx. pipiens | Plasmodium relictum | Ester, AceR | Neutral | Field | [42] | ||||
| Plasmodium relictum | Ester, AceR | Neutral | Lab | [42] | |||||
| Cx. quinquefasciatus | Wuchereria bancrofti | Esterase activity | Negative | Field | [43] | ||||
| Wuchereria bancrofti | Esterase activity | Negative | Lab | [43] | |||||
| WNV | G119S, Ester | OP | Positive | Lab | [53] | ||||
| RVV | G119S, Ester | OP | Neutral | Lab | [53] | ||||
| Aedes aegypti | DENV-2 | DDT | Neutral | Lab | Heat shock | [47] | |||
| DENV-1 | Bti | Neutral | Lab | Larval densities | [48] | ||||
| Zika | V1016I, F1534C | PYR | Positive | Lab | [54] | ||||
| Sindbis | Malathion | Positive | Lab | Heat treatment | [66] | ||||
| DENV | Bti | Bti | Positive | Lab | [67] | ||||
| ZIKV | Pyriproxyfen | Neutral | Lab | [72] | |||||
| DENV-1 | CYP and GST | V1016I, F1534C | PYR | Positive | Lab | [55] | |||
| DENV | V1016I, F1534C | Negative | Field | [46] | |||||
| Ae. albopictus | DENV-2 | Deltamethrin | Negative | Lab | [49] | ||||
| Zika | Bifenthrin | Positive | Lab | [68] | |||||
| DENV | Bifenthrin | Negative | Lab | [70] | |||||
| Frankliniella occidentalis | Tomato spotted wilt orthotospovirus | Spinosad | Positive | Lab | [74] | ||||
| Tomato spotted wilt orthotospovirus | Spinosad | Neutral | Lab | [77] | |||||
| Myzus persicae | Potato Virus Y | λ-Cyhalothrin | Ace, M918L | Diethyl carbamates PYR | Positive | Lab | [76] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eggleton, P. The state of the World’s insects. Annu. Rev. Environ. Resour. 2020, 45, 61–82. [Google Scholar] [CrossRef]
- WHO. Vector-Borne Diseases; WHO: Geneva, Switzerland, 2020. Available online: https://www.who.int/en/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 5 December 2021).
- Heck, M. Insect transmission of plant pathogens: A systems biology perspective. MSystems 2018, 3, e00168-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Beerntsen, B.T.; James, A.A.; Christensen, B.M. Genetics of mosquito vector competence. Microbiol. Mol. Biol. Rev. 2000, 64, 115–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viglietta, M.; Bellone, R.; Blisnick, A.A.; Failloux, A.B. Vector specificity of arbovirus transmission. Front. Microbiol. 2021, 12, 773211. [Google Scholar] [CrossRef]
- Chan, M.; Johansson, M.A. The incubation periods of dengue viruses. PLoS ONE 2012, 7, e50972. [Google Scholar] [CrossRef]
- Gabrieli, P.; Caccia, S.; Varotto-Boccazzi, I.; Arnoldi, I.; Barbieri, G.; Comandatore, F.; Epis, S. Mosquito trilogy: Microbiota, immunity and pathogens, and their implications for the control of disease transmission. Front. Microbiol. 2021, 12, 630438. [Google Scholar] [CrossRef]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti vector competence studies: A review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef]
- Chamberlain, R.W.; Sudia, W.D. Mechanism of transmission of viruses by mosquitoes. Annu. Rev. Entomol. 1961, 6, 371–390. [Google Scholar] [CrossRef]
- Hardy, J.L.; Houk, E.J.; Kramer, L.D.; Reeves, W.C. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu. Rev. Entomol. 1983, 28, 229–262. [Google Scholar] [CrossRef]
- Franz, A.W.; Kantor, A.M.; Passarelli, A.L.; Clem, R.J. Tissue barriers to arbovirus infection in mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.D.; Ciota, A.T. Dissecting vectorial capacity for mosquito-borne viruses. Curr. Opin. Virol. 2015, 15, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Yu, X.; Wang, P.; Cheng, G. Arbovirus lifecycle in mosquito: Acquisition, propagation and transmission. Expert. Rev. Mol. Med. 2019, 21, e1. [Google Scholar] [CrossRef] [PubMed]
- Manyilizu, W.B. Pesticides, Anthropogenic Activities, History and the Health of Our Environment: Lessons from Africa. In Pesticides—Use and Misuse and Their Impact in the Environment; Larramendy, M., Soloneski, S., Eds.; IntechOpen: London, UK, 2019; pp. 1–13. Available online: https://www.intechopen.com/chapters/66189 (accessed on 15 December 2021).
- Yu, S.J. The Toxicology and Biochemistry of Insecticides; CRC Press: Boca Raton, FL, USA, 2015; pp. 31–100. ISBN 9781482210606. [Google Scholar]
- WHO. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes, 2nd ed.; WHO: Geneva, Switzerland, 2016. Available online: http://www.who.int/malaria/publications/atoz/9789241511575/en/ (accessed on 15 December 2021).
- Oppold, A.M.; Müller, R. Epigenetics: A hidden target of insecticides. In Advances in Insect Physiology; Verlinden, H., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 313–324. [Google Scholar]
- Insecticide Resistance Action Committee (IRAC). Resistance. 2019. Available online: https://www.irac-online.org/about/resistance/ (accessed on 15 December 2021).
- Hayd, R.L.N.; Carrara, L.; de Melo Lima, J.; de Almeida, N.C.V.; Lima, J.B.P.; Martins, A.J. Evaluation of resistance to pyrethroid and organophosphate adulticides and kdr genotyping in Aedes aegypti populations from Roraima, the northernmost Brazilian State. Parasites Vectors 2020, 13, 264. [Google Scholar] [CrossRef]
- Chanda, J.; Saili, K.; Phiri, F.; Stevenson, J.C.; Mwenda, M.; Chishimba, S.; Mulube, C.; Mambwe, B.; Lungu, C.; Earle, D.; et al. Pyrethroid and carbamate resistance in Anopheles funestus Giles along Lake Kariba in Southern Zambia. Am. J. Trop. Med. Hyg. 2020, 103 (Suppl. 2), 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Du, Y.; Wu, S.; Nomura, Y.; Zhu, G.; Zhorov, B.S.; Dong, K. Molecular evidence of sequential evolution of DDT- and pyrethroid-resistant sodium channel in Aedes aegypti. PLoS Negl. Trop. Dis. 2019, 13, e0007432. [Google Scholar] [CrossRef]
- Dang, K.; Doggett, S.L.; Veera Singham, G.; Lee, C.T. Insecticide resistance and resistance mechanisms in bed bugs, Cimex spp. (Hemiptera: Cimicidae). Parasites Vectors 2017, 10, 318. [Google Scholar] [CrossRef] [Green Version]
- Zalucki, M.P.; Furlong, M.J. Behavior as a mechanism of insecticide resistance: Evaluation of the evidence. Curr. Opin. Insect Sci. 2017, 21, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Ndiath, M.O. Insecticides and insecticide resistance. Methods Mol. Biol. 2019, 2013, 287–304. [Google Scholar] [CrossRef]
- Chen, M.; Du, Y.; Nomura, Y.; Zhorov, B.S.; Dong, K. Chronology of sodium channel mutations associated with pyrethroid resistance in Aedes aegypti. Arch. Insect Biochem. Physiol. 2020, 104, e21686. [Google Scholar] [CrossRef]
- Guo, D.; Luo, J.; Zhou, Y.; Xiao, H.; He, K.; Yin, C.; Xu, J.; Li, F. ACE: An efficient and sensitive tool to detect insecticide resistance-associated mutations in insect acetylcholinesterase from RNA-Seq data. BMC Bioinform. 2017, 18, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aïzoun, N.; Aïkpon, R.; Padonou, G.G.; Oussou, O.; Oké-Agbo, F.; Gnanguenon, V.; Ossè, R.; Akogbéto, M. Mixed-function oxidases and esterases associated with permethrin, deltamethrin and bendiocarb resistance in Anopheles gambiae s.l. in the south-north transect Benin, West Africa. Parasites Vectors 2013, 6, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pethuan, S.; Jirakanjanakit, N.; Saengtharatip, S.; Chareonviriyaphap, T.; Kaewpa, D.; Rongnoparut, P. Biochemical studies of insecticide resistance in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Thailand. Trop. Biomed. 2007, 24, 7–15. [Google Scholar] [PubMed]
- Che-Mendoza, A.; Penilla, R.P.; Rodriguez, D.A. Insecticide resistance and glutathione S-transferases in mosquitoes: A review. Afr. J. Biotech. 2009, 8, 1386–1397. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhatt, K.; Huang, Y.; Lin, Z.; Chen, S. Esterase is a powerful tool for the biodegradation of pyrethroid insecticides. Chemosphere 2020, 244, 125507. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.; Kay, K.; Chitnis, N.; Hastings, I.M. Modelling the impact of insecticide-based control interventions on the evolution of insecticide resistance and disease transmission. Parasites Vectors 2018, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Malla, A.; Ramalingam, S. Epidemiology, clinical features and transmission of re-emerging arboviral infection chikungunya. Asian Pac. J. Trop. Biomed. 2019, 9, 135–139. [Google Scholar] [CrossRef]
- Boudh, S.; Singh, J.S. Pesticide Contamination: Environmental Problems and Remediation Strategies. In Emerging and Eco-Friendly Approaches for Waste Management; Bharagava, R.N., Chowdhary, P., Eds.; Springer: Singapore, 2019; pp. 245–269. [Google Scholar]
- Koch, R.L.; Hodgson, E.W.; Knodel, J.J.; Varenhorst, A.J.; Potter, B.D. Management of insecticide-resistant soybean aphids in the upper midwest of the United States. J. Integr. Pest Manag. 2018, 9, 23. [Google Scholar] [CrossRef]
- Roditakis, E.; Vasakis, E.; García-Vidal, L.; Martínez-Aguirre, M.D.R.; Rison, J.L.; Haxaire-Lutun, M.O.; Nauen, R.; Tsagkarakou, A.; Bielza, P. A four-year survey on insecticide resistance and likelihood of chemical control failure for tomato leaf miner Tuta absoluta in the European/Asian region. J. Pest Sci. 2017, 91, 421–435. [Google Scholar] [CrossRef]
- Kabula, B.; Tungu, P.; Rippon, E.J.; Steen, K.; Kisinza, W.; Magesa, S.; Mosha, F.; Donnelly, M.J. A significant association between deltamethrin resistance, Plasmodium falciparum infection and the Vgsc-1014S resistance mutation in Anopheles gambiae highlights the epidemiological importance of resistance markers. Malar. J. 2016, 15, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alout, H.; Yameogo, B.; Djogbénou, L.S.; Chandre, F.; Dabiré, R.K.; Corbel, V.; Cohuet, A. Interplay between Plasmodium infection and resistance to insecticides in vector mosquitoes. J. Infect. Dis. 2014, 210, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Tchouakui, M.; Chiang, M.C.; Ndo, C.; Kuicheu, C.K.; Amvongo-Adjia, N.; Wondji, M.J.; Tchoupo, M.; Kusimo, M.O.; Riveron, J.M.; Wondji, C.S. A marker of glutathione S-transferase-mediated resistance to insecticides is associated with higher Plasmodium infection in the African malaria vector Anopheles funestus. Sci. Rep. 2019, 9, 5772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, E.; Vaselli, N.M.; Sylla, M.; Beavogui, A.H.; Orsborne, J.; Lawrence, G.; Wiegand, R.E.; Irish, S.R.; Walker, T.; Messenger, L.A. The relationship between insecticide resistance, mosquito age and malaria prevalence in Anopheles gambiae s.l. from Guinea. Sci. Rep. 2019, 9, 8846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolie, R.; Koffi, A.A.; Ahoua Alou, L.P.; Sternberg, E.D.; N’Nan-Alla, O.; Dahounto, A.; Yapo, F.H.A.; Kanh, K.M.H.; Camara, S.; Oumbouke, W.A.; et al. Evaluation of the interaction between insecticide resistance-associated genes and malaria transmission in Anopheles gambiae sensu lato in central Côte d’Ivoire. Parasites Vectors 2021, 14, 581. [Google Scholar] [CrossRef] [PubMed]
- Vézilier, J.; Nicot, A.; Gandon, S.; Rivero, A. Insecticide resistance and malaria transmission: Infection rate and oocyst burden in Culex pipiens mosquitoes infected with Plasmodium relictum. Malar. J. 2020, 9, 379. [Google Scholar] [CrossRef] [Green Version]
- McCarroll, L.; Hemingway, J. Can insecticide resistance status affect parasite transmission in mosquitoes? Insect Biochem. Mol. Biol. 2002, 32, 1345–1351. [Google Scholar] [CrossRef]
- Kristan, M.; Lines, J.; Nuwa, A.; Ntege, C.; Meek, S.R.; Abeku, T.A. Exposure to deltamethrin affects development of Plasmodium falciparum inside wild pyrethroid resistant Anopheles gambiae s.s. mosquitoes in Uganda. Parasites Vectors 2016, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Anbalagan, R.; Shukla, A.; Subramanian, V.; Srivastava, P.K.; Krishnan, J. Monitoring of insecticide resistance and exploring the presence of virus in field populations of Culex gelidus at Thiruvarur District of Tamil. J. Commun. Dis. 2021, 53, 76–83. [Google Scholar] [CrossRef]
- Stephenson, C.J.; Coatsworth, H.; Waits, C.M.; Nazario-Maldonado, N.M.; Mathias, D.K.; Dinglasan, R.R.; Lednicky, J.A. Geographic partitioning of dengue virus transmission risk in Florida. Viruses 2021, 13, 2232. [Google Scholar] [CrossRef]
- Yadav, P.; Barde, P.V.; Gokhale, M.D.; Vipat, V.; Mishra, A.C.; Pal, J.K.; Mourya, D.T. Effect of temperature and insecticide stresses on Aedes aegypti larvae and their influence on the susceptibility of mosquitoes to dengue-2 virus. SE Asian J. Trop. Med. Public Health 2005, 36, 1139–1144. [Google Scholar]
- Alto, B.W.; Lord, C.C. Transstadial Effects of Bti on traits of Aedes aegypti and infection with dengue virus. PLoS Negl. Trop. Dis. 2016, 10, e0004370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Guo, Y.; Su, X.; Liu, S.; Yang, W.; Wu, Y.; Wu, K.; Yan, G.; Chen, X.G. Impact of deltamethrin-resistance in Aedes albopictus on its fitness cost and vector competence. PLoS Negl. Trop. Dis. 2021, 15, e0009391. [Google Scholar] [CrossRef]
- Ndo, C.; Kopya, E.; Irving, H.; Wondji, C. Exploring the impact of glutathione S-transferase (GST)-based metabolic resistance to insecticide on vector competence of Anopheles funestus for Plasmodium falciparum. Wellcome Open Res. 2019, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Alout, H.; Ndam, N.T.; Sandeu, M.M.; Djégbe, I.; Chandre, F.; Dabiré, R.K.; Djogbénou, L.S.; Corbel, V.; Cohuet, A. Insecticide resistance alleles affect vector competence of Anopheles gambiae s.s. for Plasmodium falciparum field isolates. PLoS ONE 2013, 8, e63849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndiath, M.O.; Cailleau, A.; Diedhiou, S.M.; Gaye, A.; Boudin, C.; Richard, V.; Trape, J.F. Effects of the kdr resistance mutation on the susceptibility of wild Anopheles gambiae populations to Plasmodium falciparum: A hindrance for vector control. Malar. J. 2014, 13, 340. [Google Scholar] [CrossRef] [Green Version]
- Atyame, C.M.; Alout, H.; Mousson, L.; Vazeille, M.; Diallo, M.; Weill, M.; Failloux, A.B. Insecticide resistance genes affect Culex quinquefasciatus vector competence for West Nile virus. Proc. Biol. Sci. 2019, 286, 20182273. [Google Scholar] [CrossRef] [Green Version]
- Parker-Crockett, C.; Connelly, C.R.; Siegfried, B.; Alto, B. Influence of pyrethroid resistance on vector competency for Zika virus by Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2021, 58, 1908–1916. [Google Scholar] [CrossRef]
- Chen, T.Y.; Smartt, C.T.; Shin, D. Permethrin resistance in Aedes aegypti affects aspects of vectorial capacity. Insects 2021, 12, 71. [Google Scholar] [CrossRef]
- Howard, A.F.; Koenraadt, C.J.; Farenhorst, M.; Knols, B.G.; Takken, W. Pyrethroid resistance in Anopheles gambiae leads to increased susceptibility to the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Malar. J. 2010, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Saddler, A.; Burda, P.C.; Koella, J.C. Resisting infection by Plasmodium berghei increases the sensitivity of the malaria vector Anopheles gambiae to DDT. Malar. J. 2015, 14, 134. [Google Scholar] [CrossRef] [Green Version]
- Barreaux, A.M.G.; Barreaux, P.; Thievent, K.; Koella, J.C. Larval environment influences vector competence of the malaria mosquito Anopheles gambiae. Malar. World J. 2016, 7, 1–6. Available online: https://malariaworld.org/mwj/2016/research-larval-environment-influences-vector-competence-malaria-mosquito-anopheles-gambiae (accessed on 20 January 2022).
- Cirimotich, C.M.; Ramirez, J.L.; Dimopoulos, G. Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe 2011, 10, 307–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namias, A.; Jobe, N.B.; Paaijmans, K.P.; Huijben, S. The need for practical insecticide-resistance guidelines to effectively inform mosquito-borne disease control programs. Elife 2021, 10, e65655. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Ross, P. Rates and patterns of laboratory adaptation in (mostly) insects. J. Econ. Entomol. 2018, 111, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhao, L.; Atoni, E.; Zeng, W.; Hu, X.; Matthijnssens, J.; Yuan, Z.; Xia, H. Stability of the virome in lab- and field-collected Aedes albopictus mosquitoes across different developmental stages and possible core viruses in the publicly available virome data of Aedes mosquitoes. MSystems 2020, 5, e00640-20. [Google Scholar] [CrossRef]
- Gazzoni Araújo Gonçalves, G.; Feitosa, A.P.S.; Portela-Júnior, N.C.; de Oliveira, C.M.F.; de Lima Filho, J.L.; Brayner, F.A.; Alves, L.C. Use of MALDI-TOF MS to identify the culturable midgut microbiota of laboratory and wild mosquitoes. Acta Trop. 2019, 200, 105174. [Google Scholar] [CrossRef]
- Aguilar, R.; Dong, Y.; Warr, E.; Dimopoulos, G. Anopheles infection responses; laboratory models versus field malaria transmission systems. Acta Trop. 2005, 95, 285–291. [Google Scholar] [CrossRef]
- Cornet, S.; Gandon, S.; Rivero, A. Patterns of phenoloxidase activity in insecticide resistant and susceptible mosquitoes differ between laboratory-selected and wild-caught individuals. Parasites Vectors 2013, 6, 315. [Google Scholar] [CrossRef] [Green Version]
- Muturi, E.J.; Alto, B.W. Larval environmental temperature and insecticide exposure alter Aedes aegypti competence for arboviruses. Vector-Borne Zoonotic Dis. 2011, 11, 1157–1163. [Google Scholar] [CrossRef]
- Moltini-Conclois, I.; Stalinski, R.; Tetreau, G.; Després, L.; Lambrechts, L. Larval exposure to the bacterial insecticide bti enhances dengue virus susceptibility of adult Aedes aegypti mosquitoes. Insects 2018, 9, 193. [Google Scholar] [CrossRef] [Green Version]
- Knecht, H.; Richards, S.L.; Balanay, J.A.G.; White, A.V. Impact of mosquito age and insecticide exposure on susceptibility of Aedes albopictus (Diptera: Culicidae) to infection with Zika virus. Pathogens 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellone, R.; Failloux, A.B. The Role of Temperature in shaping mosquito-borne viruses transmission. Front. Microbiol. 2020, 11, 584846. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; White, A.V.; Balanay, J.A.G. Potential for sublethal insecticide exposure to impact vector competence of Aedes albopictus (Diptera: Culicidae) for dengue and Zika viruses. Res. Rep. Trop. Med. 2017, 8, 53–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, G.; Thiévent, K.; Koella, J.C. Consequences of larval competition and exposure to permethrin for the development of the rodent malaria Plasmodium berghei in the mosquito Anopheles gambiae. Parasites Vectors 2020, 13, 107. [Google Scholar] [CrossRef]
- Alomar, A.A.; Eastmond, B.H.; Alto, B.W. The effects of exposure to pyriproxyfen and predation on Zika virus infection and transmission in Aedes aegypti. PLoS Negl. Trop. Dis. 2020, 14, e0008846. [Google Scholar] [CrossRef]
- Pigeault, R.; Nicot, A.; Gandon, S.; Rivero, A. Mosquito age and avian malaria infection. Malar. J. 2015, 14, 383. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Zheng, X.; Xu, B.; Xie, W.; Wang, S.; Zhang, Y.; Zhou, X.; Wu, Q. Insecticide resistance increases the vector competence: A case study in Frankliniella occidentalis. J. Pest Sci. 2021, 94, 83–91. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, P.; Li, W.; Zhang, J.; Huang, F.; Yang, J.; Bei, Y.; Lu, Y. De novo transcriptome sequencing in Frankliniella occidentalis to identify genes involved in plant virus transmission and insecticide resistance. Genomics 2013, 101, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Fenton, B.; Salter, W.T.; Malloch, G.; Begg, G.; Anderson, E. Stopped in its tracks: How λ-cyhalothrin can break the aphid transmission of a potato potyvirus. Pest Manag. Sci. 2015, 71, 1611–1616. [Google Scholar] [CrossRef]
- Zhao, W.; Wan, Y.; Xie, W.; Xu, B.; Zhang, Y.; Wang, S.; Wei, G.; Zhou, X.; Wu, Q. Effect of spinosad resistance on transmission of tomato spotted wilt virus by the western flower thrips (Thysanoptera: Thripidae). J. Econ. Entomol. 2015, 109, 62–69. [Google Scholar] [CrossRef]
- Hussain, S.; Farooq, M.; Malik, H.J.; Amin, I.; Scheffler, B.E.; Scheffler, J.A.; Liu, S.S.; Mansoor, S. Whole genome sequencing of Asia II 1 species of whitefly reveals that genes involved in virus transmission and insecticide resistance have genetic variances between Asia II 1 and MEAM1 species. BMC Genom. 2019, 20, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juache-Villagrana, A.E.; Pando-Robles, V.; Garcia-Luna, S.M.; Ponce-Garcia, G.; Fernandez-Salas, I.; Lopez-Monroy, B.; Rodriguez-Sanchez, I.P.; Flores, A.E. Assessing the Impact of Insecticide Resistance on Vector Competence: A Review. Insects 2022, 13, 377. https://0-doi-org.brum.beds.ac.uk/10.3390/insects13040377
Juache-Villagrana AE, Pando-Robles V, Garcia-Luna SM, Ponce-Garcia G, Fernandez-Salas I, Lopez-Monroy B, Rodriguez-Sanchez IP, Flores AE. Assessing the Impact of Insecticide Resistance on Vector Competence: A Review. Insects. 2022; 13(4):377. https://0-doi-org.brum.beds.ac.uk/10.3390/insects13040377
Chicago/Turabian StyleJuache-Villagrana, Alan E., Victoria Pando-Robles, Selene M. Garcia-Luna, Gustavo Ponce-Garcia, Ildefonso Fernandez-Salas, Beatriz Lopez-Monroy, Iram P. Rodriguez-Sanchez, and Adriana E. Flores. 2022. "Assessing the Impact of Insecticide Resistance on Vector Competence: A Review" Insects 13, no. 4: 377. https://0-doi-org.brum.beds.ac.uk/10.3390/insects13040377