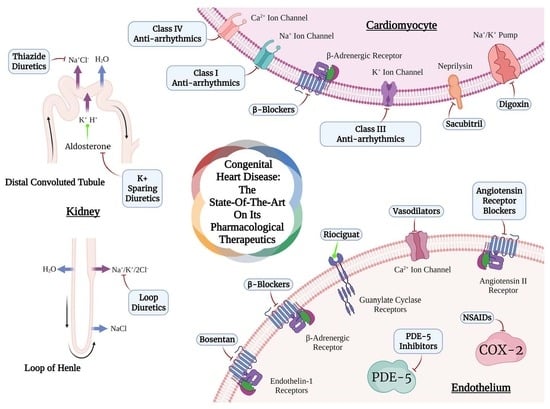
Congenital Heart Disease: The State-of-the-Art on Its Pharmacological Therapeutics
Abstract
:1. Introduction
Methodology for Literature Research
2. Drugs for CHD Treatment
2.1. Beta-Blockers
2.2. Inhibitors of Renin–Angiotensin–Aldosterone System
2.2.1. Angiotensin-Converting Enzyme Inhibitors
2.2.2. Angiotensin Receptor Blockers
2.3. Diuretics
2.3.1. Loop Diuretics
2.3.2. Thiazide Diuretics
2.3.3. Potassium-Sparing Diuretics (Mineralocorticoid Antagonists)
2.4. Vasodilators
2.4.1. Endothelin-1 Receptor Antagonists
2.4.2. Phosphodiesterase Inhibitors
2.4.3. Prostaglandins (PGs)
2.4.4. Stimulators of Soluble Guanylate Cyclase
2.5. Other Pharmacological Options for CHD Treatment
2.5.1. Angiotensin Receptor-Neprilysin Inhibitors
2.5.2. Antiarrhythmics
| Drug for CHD | Indication | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Antiarrhythmics | Atrial fibrillation rate and rhythm control, supraventricular tachycardia in adults with CHD, ventricular arrhythmias, and Wolff–Parkinson–White syndrome | -Class Ia: Procainamide: 500–1250 mg q6h oral; 15 mg/kg IV -Class Ib: Mexiletine: 150–250 mg q8h oral -Class Ic: Flecainide: 50–150 mg q12h oral -Class III: Amiodarone: ≤200 mg/d Sotalol: Initial: 80 mg q12h Increase to 160 mg q12h (max 320 mg) oral -Class IV: Diltiazem: 1.5–2 to 3–5 mg/kg/d | -QT prolongation: class I, III, and IV -Torsades de pointes: class IV -Contraindicated in structural disease: quinidine (class Ia), propafenone, and flecainide (class Ic) | [103,121,140,141] |
Class I
Class II
Class III
Class IV
Other Relevant Classes
2.5.3. Digoxin
2.5.4. Non-Steroidal Anti-Inflammatory Drugs
3. Recent Clinical Trials Testing Drugs for CHD Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soares, A.M. Mortality for critical congenital heart diseases and associated risk factors in newborns. A cohort study. Arq. Bras. Cardiol. 2018, 111, 674–675. [Google Scholar] [CrossRef]
- Wu, W.; He, J.; Shao, X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine (Baltimore) 2020, 99, e20593. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Yang, T.; Huang, P.; Wang, L.; Zhao, L.; Zhang, S.; Ye, Z.; Chen, L.; Zheng, Z.; et al. Congenital Heart Disease and Risk of Cardiovascular Disease: A Meta-Analysis of Cohort Studies. J. Am. Heart Assoc. 2019, 8, 17–24. [Google Scholar] [CrossRef]
- Bakker, M.K.; Bergman, J.E.H.; Krikov, S.; Amar, E.; Cocchi, G.; Cragan, J.; De Walle, H.E.K.; Gatt, M.; Groisman, B.; Liu, S.; et al. Prenatal diagnosis and prevalence of critical congenital heart defects: An international retrospective cohort study. BMJ Open 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, M.S.; Smith, A.G.C.; Sable, C.A.; Echko, M.M.; Wilner, L.B.; Olsen, H.E.; Atalay, H.T.; Awasthi, A.; Bhutta, Z.A.; Boucher, J.L.A.; et al. Global, regional, and national burden of congenital heart disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc. Heal. 2020, 4, 185–200. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, S.; Zühlke, L.; Babu-Narayan, S.V.; Black, G.C.; Choy, M.K.; Li, N.; Keavney, B.D. Global prevalence of congenital heart disease in school-age children: A meta-analysis and systematic review. BMC Cardiovasc. Disord. 2020, 20, 488. [Google Scholar] [CrossRef]
- Mandalenakis, Z.; Giang, K.W.; Eriksson, P.; Liden, H.; Synnergren, M.; Wåhlander, H.; Fedchenko, M.; Rosengren, A.; Dellborg, M. Survival in children with congenital heart disease: Have we reached a peak at 97%? J. Am. Heart Assoc. 2020, 9. [Google Scholar] [CrossRef]
- Chessa, M.; Baumgartner, H.; Michel-Behnke, I.; Berger, F.; Budts, W.; Eicken, A.; Søndergaard, L.; Stein, J.; Wiztsemburg, M.; Thomson, J. ESC Working Group Position Paper. Eur. Heart J. 2019, 40, 1043–1048. [Google Scholar] [CrossRef]
- Bertaud, S.; Lloyd, D.F.A.; Laddie, J.; Razavi, R. The importance of early involvement of paediatric palliative care for patients with severe congenital heart disease. Arch. Dis. Child. 2017, 102, 984–987. [Google Scholar] [CrossRef]
- Sun, R.R.; Liu, M.; Lu, L.; Zheng, Y.; Zhang, P. Congenital Heart Disease: Causes, Diagnosis, Symptoms, and Treatments. Cell Biochem. Biophys. 2015, 72, 857–860. [Google Scholar] [CrossRef]
- Shivananda, S.; Kirsh, J.; Whyte, H.E.; Muthalally, K.; McNamara, P.J. Accuracy of clinical diagnosis and decision to commence intravenous prostaglandin E1 in neonates presenting with hypoxemia in a transport setting. J. Crit. Care 2010, 25, 174.e1–174.e9. [Google Scholar] [CrossRef]
- Alonso-Gonzalez, R.; Escribano-Subías, P. Pulmonary Vasodilators in Patients with Pulmonary Arterial Hypertension Related to Congenital Heart Disease. In Pulmonary Hypertension in Adult Congenital Heart Disease; Sringer: Cham, Switzerland, 2017; pp. 253–266. [Google Scholar] [CrossRef]
- Lewis, A.B.; Chabot, M. The effect of treatment with angiotensin-converting enzyme inhibitors on survival of pediatric patients with dilated cardiomyopathy. Pediatr. Cardiol. 1993, 14, 9–12. [Google Scholar] [CrossRef]
- Ahmed, H.; VanderPluym, C. Medical management of pediatric heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 323–335. [Google Scholar] [CrossRef]
- Brida, M.; Gatzoulis, M.A. Adult congenital heart disease: Past, present and future. Acta Paediatr. Int. J. Paediatr. 2019, 108, 1757–1764. [Google Scholar] [CrossRef] [Green Version]
- Santens, B.; Van de Bruaene, A.; de Meester, P.; D’Alto, M.; Reddy, S.; Bernstein, D.; Koestenberger, M.; Hansmann, G.; Budts, W. Diagnosis and treatment of right ventricular dysfunction in congenital heart disease. Cardiovasc. Diagn. Ther. 2020, 10. [Google Scholar] [CrossRef]
- Baumgartner, H.; de Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Iung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Banach, M.; Maciejewski, M.; Bielecka-Dabrowa, A. Heart Failure Risk Predictions and Prognostic Factors in Adults with Congenital Heart Diseases. Front. Cardiovasc. Med 2022, 9, 692815. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- Zaragoza-Macias, E.; Zaidi, A.N.; Dendukuri, N.; Marelli, A. Medical Therapy for Systemic Right Ventricles: A Systematic Review (Part 1) for the 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease A Report of the American College of Cardiology/American Heart Association Task Force on C. Circulation 2019, 139, E801–E813. [Google Scholar] [CrossRef]
- Gallego, P.; Oliver, J.M. Medical therapy for heart failure in adult congenital heart disease: Does it work? Heart 2020, 106, 154–162. [Google Scholar] [CrossRef]
- Mittal, K. Pediatric Heart Failure. J. Pediatr. Crit. Care 2020, 7, 147–151. [Google Scholar] [CrossRef]
- Dézsi, C.A.; Szentes, V. The Real Role of β-Blockers in Daily Cardiovascular Therapy. Am. J. Cardiovasc. Drugs 2017, 17, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Yutzey, K.E. Cytokinesis, Beta-Blockers, and Congenital Heart Disease. N. Engl. J. Med. 2020, 382, 291–293. [Google Scholar] [CrossRef]
- Kaley, V.R.; Aregullin, E.O.; Samuel, B.P.; Vettukattil, J.J. Trends in the off-label use of β-blockers in pediatric patients. Pediatr. Int. 2019, 61, 1071–1080. [Google Scholar] [CrossRef]
- Oliver, E.; Mayor, F., Jr.; D’Ocon, P. Beta-blockers: Historical Perspective and Mechanisms of Action. Rev. Española Cardiol. 2019, 72, 853–862. [Google Scholar] [CrossRef]
- Towbin, J.A. Preface: Heart Failure in Children. Heart Fail. Clin. 2010, 6, xvii–xviii. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Ghati, N.; Ahuja, R.; Bhatt, K.; Sati, H.; Saxena, A.; Kothari, S. Efficacy and safety of propranolol in infants with heart failure due to moderate-to-large ventricular septal defect (VSD-PHF study)—A prospective randomized trial. Ann. Pediatr. Cardiol. 2021, 14, 331–340. [Google Scholar] [CrossRef]
- Salas Del Campo, P.; González, C.; Carrillo, D.; Bolte, L.; Aglony, M.; Peredo, S.; Ibarra, X.; Rojo, A.; Delucchi, A.; Pinto, V.; et al. Blood hypertension in children. Guideliness for diagnosis and treatment.: Part 2 pediatric nephrology branch, chilean pediatric society. Rev. Chil. Pediatr. 2019, 90, 336–342. [Google Scholar] [CrossRef] [Green Version]
- Schranz, D.; Voelkel, N.F. “Nihilism” of chronic heart failure therapy in children and why effective therapy is withheld. Eur. J. Pediatr. 2016, 175, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Albers, S.; Meibohm, B.; Mir, T.S.; Läer, S. Population pharmacokinetics and dose simulation of carvedilol in paediatric patients with congestive heart failure. Br. J. Clin. Pharmacol. 2008, 65, 511–522. [Google Scholar] [CrossRef] [Green Version]
- Miyake, C.Y.; Kim, J.J.; Tosur, M.; Howard, T.S.; Pham, T.D.N.; Valdes, S.O. Severe Hypoglycemia Associated With Oral Sotalol Use in Two Children. Hear. Case Rep. 2021, 7, 418–421. [Google Scholar] [CrossRef]
- Wiysonge, C.S.; Bradley, H.A.; Volmink, J.; Mayosi, B.M.; Opie, L.H. Beta-blockers for hypertension. Cochrane Database Syst. Rev. 2017, 2017, CD002003. [Google Scholar] [CrossRef] [Green Version]
- Do Vale, G.T.; Ceron, C.S.; Gonzaga, N.A.; Simplicio, J.A.; Padovan, J.C. Three Generations of β-blockers: History, Class Differences and Clinical Applicability. Curr. Hypertens. Rev. 2018, 15, 22–31. [Google Scholar] [CrossRef]
- Grandi, E.; Ripplinger, C.M. Antiarrhythmic mechanisms of beta blocker therapy. Pharmacol. Res. 2019, 146, 104274. [Google Scholar] [CrossRef]
- Vonder Muhll, I.; Liu, P.; Webb, G. Applying standard therapies to new targets: The use of ACE inhibitors and B-blockers for heart failure in adults with congenital heart disease. Int. J. Cardiol. 2004, 97, 25–33. [Google Scholar] [CrossRef]
- Recla, S.; Schmidt, D.; Logeswaran, T.; Esmaeili, A.; Schranz, D. Pediatric heart failure therapy: Why β1-receptor blocker, tissue ACE-I and mineralocorticoid-receptor-blocker? Transl. Pediatr. 2019, 8, 127–132. [Google Scholar] [CrossRef]
- Srinivasan, A. Propranolol: A 50-year historical perspective. Ann. Indian Acad. Neurol. 2019, 22, 21. [Google Scholar] [CrossRef]
- Hyman, D.A.; Siebert, V.R.; Birnbaum, G.D.; Alam, M.; Birnbaum, Y. A Modern History RAAS Inhibition and Beta Blockade for Heart Failure to Underscore the Non-equivalency of ACEIs and ARBs. Cardiovasc. Drugs Ther. 2020, 34, 215–221. [Google Scholar] [CrossRef]
- Ozgeyik, M.; Yildirim, O.T.; Kuyumcu, M.S.; Astarcioglu, M.A. A dilemma for women: Having many children risks deterioration of diastolic functions. Clin. Exp. Obstet. Gynecol. 2021, 48, 550–554. [Google Scholar] [CrossRef]
- Trinidad-Calderón, P.A.; Acosta-Cruz, E.; Rivero-Masante, M.N.; Díaz-Gómez, J.L.; García-Lara, S.; López-Castillo, L.M. Maize bioactive peptides: From structure to human health. J. Cereal Sci. 2021, 100, 103232. [Google Scholar] [CrossRef]
- Sun, H.J. Current Opinion for Hypertension in Renal Fibrosis. In Advances in Experimental Medicine and Biology; Sringer: Berlin/Heidelberg, Germany, 2019; Volume 1165. [Google Scholar]
- Das, B.B. Current state of pediatric heart failure. Children 2018, 5, 88. [Google Scholar] [CrossRef] [Green Version]
- Stotter, B.R.; Ferguson, M.A. Should ACE inhibitors and ARBs be used in combination in children? Pediatr. Nephrol. 2019, 34, 1521–1532. [Google Scholar] [CrossRef]
- Mori, Y.; Nakazawa, M.; Tomimatsu, H.; Momma, K. Long-term effect of angiotensin-converting enzyme inhibitor in volume overloaded heart during growth: A controlled pilot study. J. Am. Coll. Cardiol. 2000, 36, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Price, J.F. Congestive heart failure in children. Pediatr. Rev. 2019, 40, 60–70. [Google Scholar] [CrossRef]
- Roche, S.L.; Timberlake, K.; Manlhiot, C.; Balasingam, M.; Wilson, J.; George, K.; Mccrindle, B.W.; Kantor, P.F. Angiotensin-Converting Enzyme Inhibitor Initiation and Dose Uptitration in Children With Cardiovascular Disease: A Retrospective Review of Standard Clinical Practice and a Prospective Randomized Clinical Trial. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Momma, K. ACE inhibitors in pediatric patients with heart failure. Pediatr. Drugs 2006, 8, 55–69. [Google Scholar] [CrossRef]
- Siddiqi, N.; Shatat, I.F. Antihypertensive agents: A long way to safe drug prescribing in children. Pediatr. Nephrol. 2020, 35, 2049–2065. [Google Scholar] [CrossRef] [Green Version]
- Das, B.B.; Moskowitz, W.B.; Butler, J. Current and future drug and device therapies for pediatric heart failure patients: Potential lessons from adult trials. Children 2021, 8, 322. [Google Scholar] [CrossRef]
- Jayaprasad, N. Heart Failure in Children. Hear. Views 2016, 17, 92–99. [Google Scholar] [CrossRef]
- Goodfriend, T.L. Angiotensin receptors: History and mysteries. Am. J. Hypertens. 2000, 13, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Omboni, S.; Volpe, M. Angiotensin Receptor Blockers Versus Angiotensin Converting Enzyme Inhibitors for the Treatment of Arterial Hypertension and the Role of Olmesartan. Adv. Ther. 2019, 36, 278–297. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.; Andersen, A.; Nielsen-Kudsk, J.E. The renin-angiotensin-aldosterone-system and right heart failure in congenital heart disease. IJC Hear. Vasc. 2016, 11, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Utamayasa, A.; Rahman, M.A.; Ontoseno, T. Budiono Comparison of angiotensin-converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) for heart failure treatment in congenital heart diseases with left-to-right shunt. Indones. Biomed. J. 2020, 12, 62–68. [Google Scholar] [CrossRef]
- Shen, Y.H.; LeMaire, S.A. Molecular pathogenesis of genetic and sporadic aortic aneurysms and dissections. Curr. Probl. Surg. 2017, 54, 95–155. [Google Scholar] [CrossRef]
- Sabanayagam, A.; Cavus, O.; Williams, J.; Bradley, E. Management of Heart Failure in Adult Congenital Heart Disease. Heart Fail. Clin. 2018, 14, 569–577. [Google Scholar] [CrossRef]
- Friedberg, M.K.; Reddy, S. Right ventricular failure in congenital heart disease. Curr. Opin. Pediatr. 2019, 31, 604–610. [Google Scholar] [CrossRef]
- Felker, G.M. Must i keep taking all these medicines? Optimizing diuretics in chronic heart failure. Eur. Heart J. 2019, 40, 3613–3615. [Google Scholar] [CrossRef]
- Price, J.F.; Younan, S.; Cabrera, A.G.; Denfield, S.W.; Tunuguntla, H.A.R.I.; Choudhry, S.; Dreyer, W.J.; Akcan-Arikan, A. Diuretic Responsiveness and Its Prognostic Significance in Children With Heart Failure. J. Card. Fail. 2019, 25, 941–947. [Google Scholar] [CrossRef]
- Bua, S.; Nocentini, A.; Supuran, C.T. Carbonic Anhydrase Inhibitors as Diuretics; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128164761. [Google Scholar]
- Hsu, D.T.; Pearson, G.D. Heart failure in children part II: Diagnosis, treatment, and future directions. Circ. Hear. Fail. 2009, 2, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Mentz, R.J.; Buggey, J.; Fiuzat, M.; ErsbØll, M.K.; Schulte, P.J.; DeVore, A.D.; Eisenstein, E.L.; Anstrom, K.J.; O’Connor, C.M.; Velazquez, E.J. Torsemide versus furosemide in heart failure patients: Insights from duke university hospital. J. Cardiovasc. Pharmacol. 2015, 65, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H. Frusemide in heart failure of infancy. Arch. Dis. Child. 1971, 46, 520–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.H.; Rascati, K.L.; Lopez, K.N.; Moffett, B.S. Increased Fracture Risk with Furosemide Use in Children with Congenital Heart Disease. J. Pediatr. 2018, 199, 92–98.e10. [Google Scholar] [CrossRef] [PubMed]
- Ricci, Z.; Haiberger, R.; Pezzella, C.; Garisto, C.; Favia, I.; Cogo, P. Furosemide versus ethacrynic acid in pediatric patients undergoing cardiac surgery: A randomized controlled trial. Crit. Care 2015, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, J.M. The Long and Winding Road: Loop Diuretics in Neonatology. J. Pediatr. 2021, 231, 31–32. [Google Scholar] [CrossRef]
- Kaemmerer, H.; Apitz, C.; Brockmeier, K.; Eicken, A.; Gorenflo, M.; Hager, A.; de Haan, F.; Huntgeburth, M.; Kozlik-Feldmann, R.G.; Miera, O.; et al. Pulmonary hypertension in adults with congenital heart disease: Updated recommendations from the Cologne Consensus Conference 2018. Int. J. Cardiol. 2018, 272, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Shahin, M.H.; Johnson, J.A. Mechanisms and pharmacogenetic signals underlying thiazide diuretics blood pressure response. Curr. Opin. Pharmacol. 2016, 27, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, R.M.; Soleimani, M. Mechanism of Thiazide Diuretic Arterial Pressure Reduction: The Search Continues. Front. Pharmacol. 2019, 10, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Casas, J.C.L. Perspectivas históricas y contemporáneas de los diuréticos y su rol en la insuficiencia cardíaca A 50 años de la aparición de la furosemida. Parte 1. Un poco de historia. Insufic. Card. 2015, 10, 92–98. [Google Scholar]
- Algarni, A.; Almutairi, W.; AlQurashi, A.; Alshehrani, E.; Almrzouqi, W.; Alhazmi, H. Uses of diuretics in heart failure: A brief review. Int. J. Med. Dev. Ctries. 2020, 4, 509–512. [Google Scholar] [CrossRef]
- Brida, M.; Diller, G.P.; Gatzoulis, M.A. Systemic Right Ventricle in Adults with Congenital Heart Disease. Circulation 2018, 137, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Safdar, Z.; Frost, A.; Basant, A.; Deswal, A.; O’Brian Smith, E.; Entman, M. Spironolactone in pulmonary arterial hypertension: Results of a cross-over study. Pulm. Circ. 2020, 10, 2045894019898030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Castillo, S.; Shaddy, R.E.; Kantor, P.F. Update on pediatric heart failure. Curr. Opin. Pediatr. 2019, 31, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Malakootian, M.; Maleki, M.; Mohammadian, N.; Arabian, M. Eplerenone Reverses Age-Dependent Cardiac Fibrosis through Downregulating Osteopontin. Res. Sq. 2021, 1–12. [Google Scholar] [CrossRef]
- Esmaeiili, A.; Schranz, D. Pharmacological Chronic Heart Failure Therapy in Children. Focus on Differentiated Medical Drug Support. Cardiol. Cardiovasc. Med. 2020, 04, 432–442. [Google Scholar] [CrossRef]
- Okano, S.; Sugimoto, M.; Takase, M.; Iseki, K.; Kajihama, A.; Azuma, H. Effectiveness of high-dose spironolactone therapy in a patient with recurrent protein-losing enteropathy after the fontan procedure. Intern. Med. 2016, 55, 1611–1614. [Google Scholar] [CrossRef] [Green Version]
- Mazza, G.A.; Gribaudo, E.; Agnoletti, G. The pathophysiology and complications of fontan circulation. Acta Biomed. 2021, 92, e2021260. [Google Scholar] [CrossRef]
- Mahle, W.T.; Wang, A.; Quyyumi, A.A.; McConnell, M.E.; Book, W.M. Impact of spironolactone on endothelial function in patients with single ventricle heart. Congenit. Heart Dis. 2009, 4, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.; Lee, M.D.; McCarron, J.G. Acetylcholine released by endothelial cells facilitates flow-mediated dilatation. J. Physiol. 2016, 594, 7267–7307. [Google Scholar] [CrossRef]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Yu, X.; Ge, L.; Niu, L.; Lian, X.; Ma, H.; Pang, L. The dual role of inducible nitric oxide synthase in myocardial ischemia/reperfusion injury: Friend or foe? Oxid. Med. Cell. Longev. 2018, 2018, 8364848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.L.; Ritter, J.K. Role of nitric oxide in the cardiovascular and renal systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, K.E. PDE5 inhibitors – pharmacology and clinical applications 20 years after sildenafil discovery. Br. J. Pharmacol. 2018, 175, 2554–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowich, M.; Choudhary, G. Endothelin-1 levels and cardiovascular events. Trends Cardiovasc. Med. 2020, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Belge, C.; Delcroix, M. Treatment of pulmonary arterial hypertension with the dual endothelin receptor antagonist macitentan: Clinical evidence and experience. Ther. Adv. Respir. Dis. 2019, 13, 1–13. [Google Scholar] [CrossRef]
- Pascall, E.; Tulloh, R.M.R. Pulmonary hypertension in congenital heart disease. Future Cardiol. 2018, 14, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Diller, G.P.; Dimopoulos, K.; Kaya, M.G.; Harries, C.; Uebing, A.; Li, W.; Koltsida, E.; Simon R Gibbs, J.; Gatzoulis, M.A. Long-term safety, tolerability and efficacy of bosentan in adults with pulmonary arterial hypertension associated with congenital heart disease. Heart 2007, 93, 974–976. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, T. Inadequate Dosage May Lead to the Recurrence of Postoperative Pulmonary Hypertension in Patients With Congenital Heart Disease. Front. Pharmacol. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Apostolopoulou, S.C.; Manginas, A.; Cokkinos, D.V.; Rammos, S. Long-term oral bosentan treatment in patients with pulmonary arterial hypertension related to congenital heart disease: A 2-year study. Heart 2007, 93, 350–354. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Du, J. Bosentan for Treatment of Pediatric Idiopathic Pulmonary Arterial Hypertension: State-of-the-Art. Front. Pediatr. 2019, 7, 302. [Google Scholar] [CrossRef]
- Sitbon, O.; Beghetti, M.; Petit, J.; Iserin, L.; Humbert, M.; Gressin, V.; Simonneau, G. Bosentan for the treatment of pulmonary arterial hypertension associated with congenital heart defects. Eur. J. Clin. Investig. 2006, 36, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Skoro-Sajer, N.; Gerges, C.; Balint, O.H.; Kohalmi, D.; Kaldararova, M.; Simkova, I.; Jakowitsch, J.; Gabriel, H.; Baumgartner, H.; Gerges, M.; et al. Subcutaneous treprostinil in congenital heart disease-related pulmonary arterial hypertension. Heart 2018, 104, 1195–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Liu, J.; Zhang, R.; Zhao, M.; Wu, Y. The efficacy of bosentan combined with vardenafil in the treatment of postoperative pulmonary hypertension in children with congenital heart disease: A protocol of randomized controlled trial. Medicine (Baltimore) 2021, 100, e23896. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, B.J.; Schultze, B. Review of evidence for bosentan therapy for treatment of Eisenmenger syndrome. J. Am. Assoc. Nurse Pract. 2019, 31, 72–77. [Google Scholar] [CrossRef]
- Beghetti, M.; Haworth, S.G.; Bonnet, D.; Barst, R.J.; Acar, P.; Fraisse, A.; Ivy, D.D.; Jais, X.; Schulze-Neick, I.; Galiè, N.; et al. Pharmacokinetic and clinical profile of a novel formulation of bosentan in children with pulmonary arterial hypertension: The FUTURE-1 study. Br. J. Clin. Pharmacol. 2009, 68, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Durongpisitkul, K.; Chungsomprasong, P.; Vijarnsorn, C.; Chanthong, P.; Kanjanauthai, S.; Soongswang, J. Improved low-risk criteria scores for combination therapy of sildenafil and generic bosentan in patients with congenital heart disease with severe pulmonary hypertension: A prospective open label study. JRSM Cardiovasc. Dis. 2021, 10, 204800402098221. [Google Scholar] [CrossRef]
- Blok, I.M.; van Riel, A.C.M.J.; van Dijk, A.P.J.; Mulder, B.J.M.; Bouma, B.J. From bosentan to macitentan for pulmonary arterial hypertension and adult congenital heart disease: Further improvement? Int. J. Cardiol. 2017, 227, 51–52. [Google Scholar] [CrossRef]
- Ahmed, W.S.; Geethakumari, A.M.; Biswas, K.H. Phosphodiesterase 5 (PDE5): Structure-function regulation and therapeutic applications of inhibitors. Biomed. Pharmacother. 2021, 134, 111128. [Google Scholar] [CrossRef]
- Hutchings, D.C.; Anderson, S.G.; Caldwell, J.L.; Trafford, A.W. Phosphodiesterase-5 inhibitors and the heart: Compound cardioprotection? Heart 2018, 104, 1244–1250. [Google Scholar] [CrossRef]
- Cohen, J.L.; Nees, S.N.; Valencia, G.A.; Rosenzweig, E.B.; Krishnan, U.S. Sildenafil Use in Children with Pulmonary Hypertension. J. Pediatr. 2019, 205, 29–34.e1. [Google Scholar] [CrossRef]
- Opina, A.D.; Franklin, W.J. Management of Heart Failure in Adult Congenital Heart Disease. Prog. Cardiovasc. Dis. 2018, 61, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Jeremiasen, I.; Tran-Lundmark, K.; Idris, N.; Tran, P.K.; Moledina, S. Pulmonary Vasodilator Therapy in Children with Single Ventricle Physiology: Effects on Saturation and Pulmonary Arterial Pressure. Pediatr. Cardiol. 2020, 41, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Márquez-González, H.; Ríos, D.I.; Jean Tron, M.G.; Barajas-Nava, L.A. Use of sildenafil for pulmonary hypertension in neonates. Bol. Med. Hosp. Infant. Mex. 2020, 77, 202–206. [Google Scholar] [CrossRef]
- Yucel, I.K.; Cevik, A.; Bulut, M.O.; Dedeoʇlu, R.; Demir, I.H.; Erdem, A.; Celebi, A. Efficacy of very low-dose prostaglandin E1 in duct-dependent congenital heart disease. Cardiol. Young 2014, 25, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.C.; Vargas-Peña, M.; Pereira Dick, P.; Panizza, N.; Szwako, H.R. Uso de Prostaglandina E1 en cardiopatías congénitas ductus-dependientes. Pediatría (Asunción) 2015, 42, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Vari, D.; Xiao, W.; Behere, S.; Spurrier, E.; Tsuda, T.; Baffa, J.M. Low-dose prostaglandin E1 is safe and effective for critical congenital heart disease: Is it time to revisit the dosing guidelines? Cardiol. Young 2021, 31, 63–70. [Google Scholar] [CrossRef]
- Singh, Y.; Mikrou, P. Use of prostaglandins in duct-dependent congenital heart conditions. Arch. Dis. Child. Educ. Pract. Ed. 2018, 103, 137–140. [Google Scholar] [CrossRef]
- Akkinapally, S.; Hundalani, S.G.; Kulkarni, M.; Fernandes, C.J.; Cabrera, A.G.; Shivanna, B.; Pammi, M. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef]
- Lewis, A.B.; Freed, M.D.; Heymann, M.A.; Roehl, S.L.; Kensey, R.C. Side effects of therapy with prostaglandin E1 in infants with critical congenital heart disease. Circulation 1981, 64, 893–898. [Google Scholar] [CrossRef] [Green Version]
- Aykanat, A.; Yavuz, T.; Özalkaya, E.; Topçuoğlu, S.; Ovalı, F.; Karatekin, G. Long-Term Prostaglandin E1 Infusion for Newborns with Critical Congenital Heart Disease. Pediatr. Cardiol. 2016, 37, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Sandner, P. From molecules to patients: Exploring the therapeutic role of soluble guanylate cyclase stimulators. Biol. Chem. 2018, 399, 679–690. [Google Scholar] [CrossRef]
- Lammers, A.E.; Diller, G.P. Riociguat for pulmonary hypertension in congenital heart disease: Opportunities and challenges. Heart 2015, 101, 1771–1772. [Google Scholar] [CrossRef] [PubMed]
- Varela, D.L.; Teleb, M.; El-Mallah, W. Advanced therapies for the management of adults with pulmonary arterial hypertension due to congenital heart disease: A systematic review. Open Heart 2018, 5, e000744. [Google Scholar] [CrossRef] [Green Version]
- Klinger, J.R.; Chakinala, M.M.; Langleben, D.; Rosenkranz, S.; Sitbon, O. Riociguat: Clinical Research and Evolving Role in Therapy; NCBI: Bethesda, MD, USA, 2021; Volume 87, ISBN 0000000329. [Google Scholar]
- Khaybullina, D.; Patel, A.; Zerilli, T. Riociguat (adempas): A novel agent for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. P T 2014, 39, 749–758. [Google Scholar]
- Rosenkranz, S.; Ghofrani, H.A.; Beghetti, M.; Ivy, D.; Frey, R.; Fritsch, A.; Weimann, G.; Saleh, S.; Apitz, C. Riociguat for pulmonary arterial hypertension associated with congenital heart disease. Heart 2015, 101, 1792–1799. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, Y. Influence of riociguat treatment on pulmonary arterial hypertension: A meta-analysis of randomized controlled trials. Herz 2019, 44, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, S.; Uyeda, T.; Saito, M.; Ishii, T.; Inage, A.; Hamamichi, Y.; Yazaki, S.; Yoshikawa, T. Efficacy and Safety of Low-Dose Amiodarone Therapy for Tachyarrhythmia in Congenital Heart Disease. Pediatr. Cardiol. 2018, 39, 1016–1022. [Google Scholar] [CrossRef]
- Oster, M.E.; Kelleman, M.; McCracken, C.; Ohye, R.G.; Mahle, W.T. Association of digoxin with interstage mortality: Results from the pediatric heart network single ventricle reconstruction trial public use dataset. J. Am. Heart Assoc. 2016, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gulack, B.C.; Laughon, M.M.; Clark, R.H.; Sankar, M.N.; Hornik, C.P.; Brian Smith, P. Comparative effectiveness and safety of indomethacin versus ibuprofen for the treatment of patent ductus arteriosus. Early Hum. Dev. 2015, 91, 725–729. [Google Scholar] [CrossRef] [Green Version]
- Fala, L. Entresto (Sacubitril/valsartan): First-in-class angiotensin receptor neprilysin inhibitor FDA approved for patients with heart failure. Am. Heal. Drug Benefits 2015, 8, 330. [Google Scholar]
- Lei, M.; Wu, L.; Terrar, D.A.; Huang, C.L.H. Modernized classification of cardiac antiarrhythmic drugs. Circulation 2018, 138, 1879–1896. [Google Scholar] [CrossRef] [PubMed]
- Maideen, N. Pharmacodynamic interactions of thiazide diuretics. Int. J. Med. Dev. Ctries. 2020, 4, 1007–1010. [Google Scholar] [CrossRef]
- Sallmon, H.; Koehne, P.; Hansmann, G. Recent Advances in the Treatment of Preterm Newborn Infants with Patent Ductus Arteriosus. Clin. Perinatol. 2016, 43, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Barallat, J.; Richards, A.M. A Test in Context: Neprilysin: Function, Inhibition, and Biomarker. J. Am. Coll. Cardiol. 2016, 68, 639–653. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V. Neprilysin inhibition to treat heart failure: A tale of science, serendipity, and second chances. Eur. J. Heart Fail. 2015, 17, 242–247. [Google Scholar] [CrossRef]
- Lluri, G.; Lin, J.; Reardon, L.; Miner, P.; Whalen, K.; Aboulhosn, J. Early Experience With Sacubitril/Valsartan in Adult Patients With Congenital Heart Disease. World J. Pediatr. Congenit. Hear. Surg. 2019, 10, 292–295. [Google Scholar] [CrossRef]
- Maurer, S.J.; Pujol Salvador, C.; Schiele, S.; Hager, A.; Ewert, P.; Tutarel, O. Sacubitril/valsartan for heart failure in adults with complex congenital heart disease. Int. J. Cardiol. 2020, 300, 137–140. [Google Scholar] [CrossRef]
- Vilela-Martin, J.F. Spotlight on valsartan-sacubitril fixed-dose combination for heart failure: The evidence to date. Drug Des. Devel. Ther. 2016, 10, 1627–1639. [Google Scholar] [CrossRef] [Green Version]
- Shaddy, R.; Canter, C.; Halnon, N.; Kochilas, L.; Rossano, J.; Bonnet, D.; Bush, C.; Zhao, Z.; Kantor, P.; Burch, M.; et al. Design for the sacubitril/valsartan (LCZ696) compared with enalapril study of pediatric patients with heart failure due to systemic left ventricle systolic dysfunction (PANORAMA-HF study). Am. Heart J. 2017, 193, 23–34. [Google Scholar] [CrossRef]
- Baracco, R.; Kapur, G. Clinical utility of valsartan in the treatment of hypertension in children and adolescents. Patient Prefer. Adherence 2011, 5, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Elia, E.; Iacovoni, A.; Vaduganathan, M.; Lorini, F.L.; Perlini, S.; Senni, M. Neprilysin inhibition in heart failure: Mechanisms and substrates beyond modulating natriuretic peptides. Eur. J. Heart Fail. 2017, 19, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Mankad, P.; Kalahasty, G. Antiarrhythmic Drugs: Risks and Benefits. Med. Clin. N. Am. 2019, 103, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.K.; McGowan, M.; Smyth, A.; Wright, G.A.; Gardner, R.S. Classification and choice of antiarrhythmic therapies. Prescriber 2020, 31, 11–17. [Google Scholar] [CrossRef]
- Williams, E.M.V. A Classification of Antiarrhythmic Actions Reassessed After a Decade of New Drugs. J. Clin. Pharmacol. 1984, 24, 129–147. [Google Scholar] [CrossRef]
- Jones, B.; Burnand, C. Antiarrhythmic drugs. Anaesth. Intensive Care Med. 2021, 22, 319–323. [Google Scholar] [CrossRef]
- Vorhies, E.E.; Ivy, D.D. Drug treatment of pulmonary hypertension in children. Pediatr. Drugs 2014, 16, 43–65. [Google Scholar] [CrossRef] [Green Version]
- Contractor, T.; Levin, V.; Mandapati, R. Drug Therapy in Adult Congenital Heart Disease. Card. Electrophysiol. Clin. 2017, 9, 295–309. [Google Scholar] [CrossRef]
- Moe, T.G.; Abrich, V.A.; Rhee, E.K. Atrial Fibrillation in Patients with Congenital Heart Disease. J. Atr. Fibrillation 2017, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wasmer, K.; Eckardt, L.; Baumgartner, H.; Köbe, J. Therapy of supraventricular and ventricular arrhythmias in adults with congenital heart disease—Narrative review. Cardiovasc. Diagn. Ther. 2021, 11, 550–562. [Google Scholar] [CrossRef]
- Wasmer, K.; Eckardt, L. Management of supraventricular arrhythmias in adults with congenital heart disease. Heart 2016, 102, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Dempsey, C.E.; Hancox, J.C. The Basis for Low-affinity hERG Potassium Channel Block by Sotalol. J. Pharmacol. Pharmacother. 2017, 8, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Valdés, S.O.; Miyake, C.Y.; Niu, M.C.; de la Uz, C.M.; Asaki, S.Y.; Landstrom, A.P.; Schneider, A.E.; Rusin, C.G.; Patel, R.; Lam, W.W.; et al. Early experience with intravenous sotalol in children with and without congenital heart disease. Hear. Rhythm 2018, 15, 1862–1869. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, V.; Laredo, M.; Abadir, S.; Mondésert, B.; Khairy, P. Atrial fibrillation in adults with congenital heart disease. Int. J. Cardiol. 2019, 287, 148–154. [Google Scholar] [CrossRef]
- Hernández-Madrid, A.; Paul, T.; Abrams, D.; Aziz, P.F.; Blom, N.A.; Chen, J.; Chessa, M.; Combes, N.; Dagres, N.; Diller, G.; et al. Arrhythmias in congenital heart disease: A position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congeni. Europace 2018, 20, 1719–1720. [Google Scholar] [CrossRef]
- HS, K.; U, K. Wanted: Class VI Antiarrhythmic Drug Action; New Start for a Rational Drug Therapy. J. Hear. Health 2019, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Patocka, J.; Nepovimova, E.; Wu, W.; Kuca, K. Digoxin: Pharmacology and toxicology—A review. Environ. Toxicol. Pharmacol. 2020, 79, 1–6. [Google Scholar] [CrossRef]
- Virgadamo, S. Digoxin: A systematic review in atrial fibrillation, congestive heart failure and post myocardial infarction. World J. Cardiol. 2015, 7, 808. [Google Scholar] [CrossRef]
- Brown, D.W.; Mangeot, C.; Anderson, J.B.; Peterson, L.E.; King, E.C.; Lihn, S.L.; Neish, S.R.; Fleishman, C.; Phelps, C.; Hanke, S.; et al. Digoxin use is associated with reduced interstage mortality in patients with no history of arrhythmia after stage i palliation for single ventricle heart disease. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Klausner, R.E.; Parra, D.; Kohl, K.; Brown, T.; Hill, G.D.; Minich, L.A.; Godown, J. Impact of Digoxin Use on Interstage Outcomes of Single Ventricle Heart Disease (From a NPC-QIC Registry Analysis). Am. J. Cardiol. 2021, 154, 99–105. [Google Scholar] [CrossRef]
- Sun, H.Y. Prenatal diagnosis of congenital heart defects: Echocardiography. Transl. Pediatr. 2021, 10, 2210–2224. [Google Scholar] [CrossRef] [PubMed]
- Abdel Jalil, M.H.; Abdullah, N.; Alsous, M.M.; Saleh, M.; Abu-Hammour, K. A systematic review of population pharmacokinetic analyses of digoxin in the paediatric population. Br. J. Clin. Pharmacol. 2020, 86, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, B.; Jain, S. Digoxin in management of heart failure in children: Should it be continued or relegated to the history books? Ann. Pediatr. Cardiol. 2009, 2, 149. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.T.; Menon, S.C.; Lambert, L.M.; Burch, P.T.; Sheng, X.; Minich, L.L.; Williams, R.V. Digoxin Use in Infants with Single Ventricle Physiology: Secondary Analysis of the Pediatric Heart Network Infant Single Ventricle Trial Public Use Dataset. Pediatr. Cardiol. 2018, 39, 1200–1209. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Benitz, W.E.; Bhombal, S. The use of non-steroidal anti-inflammatory drugs for patent ductus arteriosus closure in preterm infants. Semin. Fetal Neonatal Med. 2017, 22, 302–307. [Google Scholar] [CrossRef]
- Slaughter, J.L.; Reagan, P.B.; Newman, T.B.; Klebanoff, M.A. Comparative effectiveness of nonsteroidal anti-inflammatory drug treatment vs no treatment for patent ductus arteriosus in preterm infants. JAMA Pediatr. 2017, 171, e164354. [Google Scholar] [CrossRef]
- Varga, Z.; rafay ali Sabzwari, S.; Vargova, V. Cardiovascular Risk of Nonsteroidal Anti-Inflammatory Drugs: An Under-Recognized Public Health Issue. Cureus 2017, 9, e1144. [Google Scholar] [CrossRef] [Green Version]
- Hillier, K.; Jones, K.; MacInnis, M.; Mitra, S. Comparison of standard versus high-dose ibuprofen for the treatment of hemodynamically significant patent ductus arteriosus in preterm infants. J. Perinatol. 2021, 41, 1142–1148. [Google Scholar] [CrossRef]
- Waldvogel, S.; Atkinson, A.; Wilbeaux, M.; Nelle, M.; Berger, M.R.; Gerull, R. High Dose Indomethacin for Patent Ductus Arteriosus Closure Increases Neonatal Morbidity. Am. J. Perinatol. 2021, 38, 707–713. [Google Scholar] [CrossRef]
- Van Driest, S.L.; Jooste, E.H.; Shi, Y.; Choi, L.; Darghosian, L.; Hill, K.D.; Smith, A.H.; Kannankeril, P.J.; Roden, D.M.; Ware, L.B. Association between early postoperative acetaminophen exposure and acute kidney injury in pediatric patients undergoing cardiac surgery. JAMA Pediatr. 2018, 172, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluckow, M.; Jeffery, M.; Gill, A.; Evans, N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F97. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Ibuprofen: Pharmacology, efficacy and safety. Inflammopharmacology 2009, 17, 275–342. [Google Scholar] [CrossRef] [PubMed]
- Hochwald, O.; Mainzer, G.; Borenstein-Levin, L.; Jubran, H.; Dinur, G.; Zucker, M.; Mor, M.; Khoury, A.; Kugelman, A. Adding Paracetamol to Ibuprofen for the Treatment of Patent Ductus Arteriosus in Preterm Infants: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. Am. J. Perinatol. 2018, 35, 1319–1325. [Google Scholar] [CrossRef]
- Vaidya, R.; Wilson, D.; Paris, Y.; Madore, L.; Singh, R. Use of acetaminophen for patent ductus arteriosus treatment: A single center experience. J. Matern. Neonatal Med. 2020, 33, 2723–2729. [Google Scholar] [CrossRef] [PubMed]
- Hnatiuk, A.P.; Briganti, F.; Staudt, D.W.; Mercola, M. Human iPSC modeling of heart disease for drug development. Cell Chem. Biol. 2021, 28, 271–282. [Google Scholar] [CrossRef]
- Cedars, A.M.; Kutty, S. The Way Forward in Congenital Heart Disease Research. JAMA Cardiol. 2020, 5, 979–980. [Google Scholar] [CrossRef]
- Marelli, A. The Future of Adult Congenital Heart Disease Research: Precision Health Services Delivery for the Next Decade. Can. J. Cardiol. 2019, 35, 1609–1619. [Google Scholar] [CrossRef] [Green Version]
- Pearson, G.D.; Burns, K.M.; Pemberton, V.L. Clinical Trials in Children. Princ. Pract. Clin. Trials 2020, 1–17. [Google Scholar] [CrossRef]
- Hill, K.D.; Baldwin, H.S.; Bichel, D.P.; Ellis, A.M.; Graham, E.M.; Hornik, C.P.; Jacobs, J.P.; Jaquiss, R.D.B.; Jacobs, M.L.; Kannankeril, P.J.; et al. Overcoming underpowering: Trial simulations and a global rank end point to optimize clinical trials in children with heart disease. Am. Heart J. 2020, 226, 188–197. [Google Scholar] [CrossRef]
- Bokma, J.P.; Winter, M.M.; Van Dijk, A.P.; Vliegen, H.W.; Van Melle, J.P.; Meijboom, F.J.; Post, M.C.; Berbee, J.K.; Boekholdt, S.M.; Groenink, M.; et al. Effect of losartan on right ventricular dysfunction: Results from the Double-Blind, Randomized REDEFINE Trial (Right Ventricular Dysfunction in Tetralogy of Fallot: Inhibition of the Renin-Angiotensin-Aldosterone System) in Adults with Repaired Tetralogy. Circulation 2018, 137, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, O.I.; Kuijpers, J.M.; Meijboom, F.J.; Post, M.C.; Jongbloed, M.R.M.; Duijnhouwer, A.L.; Van Dijk, A.P.J.; Van Melle, J.P.; Konings, T.C.; Zwinderman, A.H.; et al. High burden of drug therapy in adult congenital heart disease: Polypharmacy as marker of morbidity and mortality. Eur. Hear. J. Cardiovasc. Pharmacother. 2019, 5, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Van Dissel, A.C.; Mulder, B.J.M.; Bouma, B.J. The changing landscape of pulmonary arterial hypertension in the adult with congenital heart disease. J. Clin. Med. 2017, 6, 40. [Google Scholar] [CrossRef]
- McLaughlin, V.; Chin, K.; Kim, N.H.; Flynn, M.; Ong, R.; Wetherill, G.; Channick, R. Treatment with macitentan for pulmonary arterial hypertension (PAH) associated with congenital heart disease (CHD): Real-world experience from the combined OPUS and OrPHeUS data sets. Eur. Heart J. 2020, 41, ehaa946.2293. [Google Scholar] [CrossRef]
- Meliota, G.; Lombardi, M.; Benevento, M.; Console, V.; Ciccone, M.M.; Solarino, B.; Vairo, U. Off-Label Use of Cardiovascular Drugs in the Home Therapy of Children With Congenital or Acquired Heart Disease. Am. J. Cardiol. 2022, 166, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.P.; Arvanitaki, A.; Opotowsky, A.R.; Jenkins, K.; Moons, P.; Kempny, A.; Tandon, A.; Redington, A.; Khairy, P.; Mital, S.; et al. Lifespan Perspective on Congenital Heart Disease Research: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 2219–2235. [Google Scholar] [CrossRef]
- Hummel, J.; Rücker, G.; Stiller, B. Prophylactic levosimendan for the prevention of low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar]
- Bajcetic, M.; de Wildt, S.N.; Dalinghaus, M.; Breitkreutz, J.; Klingmann, I.; Lagler, F.B.; Keatley-Clarke, A.; Breur, J.M.; Male, C.; Jovanovic, I.; et al. Orodispersible minitablets of enalapril for use in children with heart failure (LENA): Rationale and protocol for a multicentre pharmacokinetic bridging study and follow-up safety study. Contemp. Clin. Trials Commun. 2019, 15, 100393. [Google Scholar] [CrossRef]
- Laeer, S.; Cawello, W.; Burckhardt, B.B.; Bajcetic, M.; Breur, J.M.P.J.; Dalinghaus, M.; Male, C.; De Wildt, S.N.; Breitkreutz, J.; Faisal, M.; et al. Enalapril and Enalaprilat Pharmacokinetics in Children with Heart Failure Due to Dilated Cardiomyopathy and Congestive Heart Failure after Administration of an Orodispersible Enalapril Minitablet (LENA-Studies). Pharmaceutics 2022, 14, 1163. [Google Scholar] [CrossRef]
- Loomba, R.S.; Rausa, J.; Dorsey, V.; Bronicki, R.A.; Villarreal, E.G.; Flores, S. The impact of medical interventions on admission characteristics in children with congenital heart disease and cardiomyopathy. Cardiol. Young 2021, 31, 406–413. [Google Scholar] [CrossRef]
- Bouma, B.J.; Mulder, B.J.M. Changing Landscape of Congenital Heart Disease. Circ. Res. 2017, 120, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.M.; Pemberton, V.L.; Schramm, C.A.; Pearson, G.D.; Kaltman, J.R. Trends in National Institutes of Health-Funded Congenital Heart Disease Research from 2005 to 2015. Pediatr. Cardiol. 2017, 38, 974–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinton, R.B.; Ware, S.M. Heart Failure in Pediatric Patients with Congenital Heart Disease. Circ. Res. 2017, 120, 978–994. [Google Scholar] [CrossRef] [Green Version]
- Kuang, H.Y.; Wu, Y.H.; Yi, Q.J.; Tian, J.; Wu, C.; Shou, W.N.; Lu, T.W. The efficiency of endothelin receptor antagonist bosentan for pulmonary arterial hypertension associated with congenital heart disease: A systematic review and meta-analysis. Medicine 2018, 97, e0075. [Google Scholar] [CrossRef] [PubMed]
- Foote, H.P.; Hornik, C.P.; Hill, K.D.; Rotta, A.T.; Chamberlain, R.; Thompson, E.J. A systematic review of the evidence supporting post-operative diuretic use following cardiopulmonary bypass in children with Congenital Heart Disease. Cardiol. Young 2021, 31, 699–706. [Google Scholar] [CrossRef]
- Russell, M.W.; Chung, W.K.; Kaltman, J.R.; Miller, T.A. Advances in the understanding of the genetic determinants of congenital heart disease and their impact on clinical outcomes. J. Am. Heart Assoc. 2018, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Brida, M.; Gatzoulis, M.A. Adult congenital heart disease: Past, present, future. Int. J. Cardiol. Congenit. Hear. Dis. 2020, 1, 100052. [Google Scholar] [CrossRef]


| Drug for CHD | Indication | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Beta-blockers | Left ventricle systolic dysfunction | 1st-generation: Propranolol: 4 mg/kg/d 2nd-generation: Bisoprolol 0.1–0.2 mg/kg/d 3rd generation: Carvedilol: Patients within: 28 d-23 m: 3 mg/kg 2–11 y: 2 mg/kg 12–15 y: 1 mg/kg | -Lightheadedness and dizziness -Contraindicated in asthma -Hypoglycemia in infants with sotalol use | [27,28,29,30,31,32] |
| RAAS Inhibitor for CHD | Indications | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Angiotensin-converting enzyme inhibitors (ACEIs) | Asymptomatic CHDs and symptomatic heart failure | -Captopril: Neonates: 0.4–1.6 mg/kg/d in 3 doses Infants: 0.5–4 mg/kg/d in 3 doses -Enalapril: Children > 2 y: 0.1–0.5 mg/kg/d in two doses -Lisinopril: 5 mg/d | -Acute kidney injury, angioedema, cough, hyperkalemia, and hypotension -Contraindicated in bilateral renal artery stenosis | [14,46,47,48,49] |
| RAAS Inhibitor for CHD | Indications | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Angiotensin receptor blockers (ARBs) | -Left ventricle systolic dysfunction in patients with intolerance to ACEIs -Slows the progression of genetically triggered aortopathy disease | Losartan: 25–50 mg/d Valsartan: 1.3 mg/kg/d | Acute kidney injury, diarrhea, dizziness, headache, hyperkalemia, and hypotension | [24,49,54,55,56] |
| Diuretic for CHD | Indication | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Loop Diuretics | -Decompensated heart failure -Fluid overload in CHD | Furosemide: 0.08 mg/kg/h | Hypercalciuria, nephrolithiasis, osteoporosis, and pre-renal azotemia Tolerance after chronic use | [14,65,66,67,68] |
| Diuretic for CHD | Indication | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Thiazide Diuretics | Postoperative fluid overload | -Chlorothiazide: 10 mg/kg/d -Hydro- chlorothiazide: 1–2 mg/kg/d | -Hyperglycemia, hyperlipidemia, hyperuricemia, hypokalemia, metabolic alkalosis, and prerenal azotemia -Contraindicated in patients with anuria | [26,49] |
| Diuretic for CHD | Indication | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Potassium -Sparing Diuretics | -Symptomatic heart failure, systemic right ventricle morphology, double-inlet right morphology ventricle, hypoplastic left heart syndrome, and transposition of great vessels with arterial switch operation repair | -Spironolactone: 25–75 mg/d -Eplerenone: 50 mg/d | -Anti-androgenic and estrogenic effects, gynecomastia, and hyperkalemia | [54,73,74] |
| Vasodilator for CHD | Indication | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Endothelin-1 Receptor Antagonists (ERAs) | -Adult pulmonary arterial hypertension associated with CHD -Idiopathic pulmonary hypertension -Eisenmenger syndrome | Bosentan: 2 mg/kg q12h | Dizziness, flushing, hemoptysis, increased LFTs, and non-sustained ventricular tachycardia | [89,90,91,92] |
| Vasodilator for CHD | Indication | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| PDE-5 Inhibitors | Pulmonary arterial hypertension and pulmonary hyper flow from any CHD | Sildenafil: 1 mg/kg q8h | Dizziness, lupus-like syndrome, orthostatic hypotension, peripheral edema, and refle Xtachycardia | [49,102,104] |
| Vasodilator for CHD | Indication | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Prostaglandins | Aortic, mitral, pulmonary, and tricuspid atresia, aortic stenosis, interrupted aortic arch, hypoplastic left heart syndrome, pulmonary stenosis, severe mitral stenosis, and transposition of great vessels with intact interventricular septum | PGE1: Initial dose of 0.025 µg/kg/min to 0.01 µg/kg/min | Apnea (dose-dependent), bradycardia, diarrhea, disseminated intravascular coagulation, fever, hypotension, hypothermia, and seizures | [108,109,110,111,112,113] |
| Vasodilator for CHD | Indication | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Stimulators of soluble guanylate cyclase (sCG) | Adult pulmonary arterial hypertension associated with CHD | Riociguat: 1.5–2.5 mg q8h | -Diarrhea, dizziness, dyspepsia, headache, hypertension, nausea, peripheral edema, and vomiting -Contraindicated during pregnancy | [117,118,119] |
| Drug for CHD | Indication | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Angiotensin Receptor Neprilysin Inhibitors (ARNIs) | -Symptomatic NYHA class II or III -Heart failure with systolic dysfunction | Sacubitril–valsartan: 3.1 mg/kg q12h | Renal dysfunction | [103,130,131,132,133] |
| Drug for CHD | Indication | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Digoxin | -Symptomatic heart failure -Adult and fetal tachyarrhythmias | Digoxin: 8–10 mcg/kg/24 h in children from 2 to 10 years | Atrial tachycardia, complete heart block, delirium nausea, hypomagnesemia, hypokalemia, sinoatrial/atrioventricular junction, sinus arrest, vomiting, and visual changes | [141,155,156,157] |
| Drug for CHD | Indication | Dosing Regimen | Adverse Effects/ Contraindications | Refs. |
|---|---|---|---|---|
| Non- steroidal anti- inflammatory Drugs (NSAIDs) | -Patent ductus arteriosus closure in preterm infants | -Ibuprofen (3 doses): 10–5–5 mg/kg/d -Indomethacin (3–6 doses): 0.2 mg/kg IV -Acetaminophen (3–7 d): 15 mg/kg q6h | Gastrointestinal and renal toxicity, heart failure exacerbation, and hypertension | [159,160,161,162,163,164] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela-Chinchilla, C.D.; Sánchez-Mejía, D.E.; Trinidad-Calderón, P.A. Congenital Heart Disease: The State-of-the-Art on Its Pharmacological Therapeutics. J. Cardiovasc. Dev. Dis. 2022, 9, 201. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd9070201
Varela-Chinchilla CD, Sánchez-Mejía DE, Trinidad-Calderón PA. Congenital Heart Disease: The State-of-the-Art on Its Pharmacological Therapeutics. Journal of Cardiovascular Development and Disease. 2022; 9(7):201. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd9070201
Chicago/Turabian StyleVarela-Chinchilla, Carlos Daniel, Daniela Edith Sánchez-Mejía, and Plinio A. Trinidad-Calderón. 2022. "Congenital Heart Disease: The State-of-the-Art on Its Pharmacological Therapeutics" Journal of Cardiovascular Development and Disease 9, no. 7: 201. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd9070201