A Single Session of Virtual Reality Improved Tiredness, Shortness of Breath, Anxiety, Depression and Well-Being in Hospitalized Individuals with COVID-19: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Inclusion and Exclusion Criteria
2.3. Study Closure or Discontinuity Criteria
2.4. Primary Outcome Measures
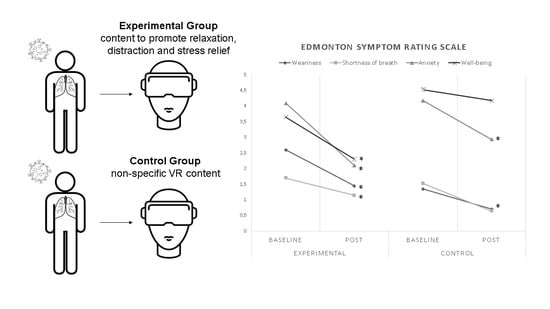
2.4.1. Edmonton Symptom Rating Scale
2.4.2. The Borg Scale for Perceived Effort
2.4.3. Hospital Anxiety and Depression Scale
2.5. Secondary Outcome Measures
2.6. Randomization, Blinding, and Allocation
2.7. Study Procedures
2.8. Equipment
2.9. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Eurosurveillance Editorial Team. Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. EuroSurveillance 2020, 25, 200131e. [Google Scholar] [CrossRef]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 5 May 2022).
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019-COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, M.D.S.; Souza, J. SARS-CoV-2: A clinical update—II. Rev. Assoc. Med. Bras. 2020, 66, 547–557. [Google Scholar] [CrossRef]
- Cornaggia, C.M.; Piscitelli, D.; Ferriero, G. COVID-19 pandemic and rehabilitation: How protective is social isolation in the care of frail patients (and their caregivers)? Eur. J. Phys. Rehabil. Med. 2021, 57, 319–320. [Google Scholar] [CrossRef]
- Moser, D.A.; Glaus, J.; Frangou, S.; Schechter, D.S. Years of life lost due to the psychosocial consequences of COVID-19 mitigation strategies based on Swiss data. Eur. Psychiatry 2020, 63, e58. [Google Scholar] [CrossRef]
- Armitage, R.; Nellums, L.B. COVID-19 and the consequences of isolating the elderly. Lancet Public Health 2020, 5, e256. [Google Scholar] [CrossRef] [Green Version]
- Piscitelli, D.; Perin, C.; Tremolizzo, L.; Peroni, F.; Cerri, C.G.; Cornaggia, C.M. Functional movement disorders in a patient with COVID-19. Neurol. Sci. 2020, 41, 2343–2344. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Mantovani, E.; Zucchella, C.; Bottiroli, S.; Federico, A.; Giugno, R.; Sandrini, G.; Chiamulera, C.; Tamburin, S. Telemedicine and Virtual Reality for Cognitive Rehabilitation: A Roadmap for the COVID-19 Pandemic. Front. Neurol. 2020, 11, 926. [Google Scholar] [CrossRef]
- Mosadeghi, S.; Reid, M.W.; Martinez, B.; Rosen, B.T.; Spiegel, B.M. Feasibility of an Immersive Virtual Reality Intervention for Hospitalized Patients: An Observational Cohort Study. JMIR Ment. Health 2016, 3, e28. [Google Scholar] [CrossRef]
- Pagliari, C.; Di Tella, S.; Jonsdottir, J.; Mendozzi, L.; Rovaris, M.; De Icco, R.; Milanesi, T.; Federico, S.; Agostini, M.; Goffredo, M.; et al. Effects of home-based virtual reality telerehabilitation system in people with multiple sclerosis: A randomized controlled trial. J. Telemed. Telecare 2021, 1357633X211054839. [Google Scholar] [CrossRef]
- Pournajaf, S.; Goffredo, M.; Pellicciari, L.; Piscitelli, D.; Criscuolo, S.; Pera, D.L.; Damiani, C.; Franceschini, M. Effect of balance training using virtual reality-based serious games in individuals with total knee replacement: A randomized controlled trial. Ann. Phys. Rehabil. Med. 2021, 101609. [Google Scholar] [CrossRef]
- Malloy, K.M.; Milling, L.S. The effectiveness of virtual reality distraction for pain reduction: A systematic review. Clin. Psychol. Rev. 2010, 30, 1011–1018. [Google Scholar] [CrossRef]
- Jones, T.; Moore, T.; Choo, J. The Impact of Virtual Reality on Chronic Pain. PLoS ONE 2016, 11, e0167523. [Google Scholar] [CrossRef]
- Chirico, A.; Lucidi, F.; De Laurentiis, M.; Milanese, C.; Napoli, A.; Giordano, A. Virtual Reality in Health System: Beyond Entertainment. A Mini-Review on the Efficacy of VR During Cancer Treatment. J. Cell. Physiol. 2016, 231, 275–287. [Google Scholar] [CrossRef]
- Hoffman, H.G.; Patterson, D.R.; Seibel, E.; Soltani, M.; Jewett-Leahy, L.; Sharar, S.R. Virtual reality pain control during burn wound debridement in the hydrotank. Clin. J. Pain 2008, 24, 299–304. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Group, C. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, 332. [Google Scholar] [CrossRef]
- Caramelli, P.; Herrera, E.J.; Nitrini, R.O. Mini-exame do estado mental no diagnóstico de demência em idosos analfabetos. Arq. Neuropsiquiatr. 1998, 56, 605–612. [Google Scholar]
- Lourenco, R.A.; Veras, R.P. Mini-Mental State Examination: Psychometric characteristics in elderly outpatients. Rev. Saude Publica 2006, 40, 712–719. [Google Scholar] [CrossRef]
- Monteiro, D.d.R. Escala de Edmonton e Cuidados Paliativos. Federal University of Rio Grande do Sul. Nursing School. 2009. Available online: http://hdl.handle.net/10183/24699 (accessed on 20 November 2020).
- Chang, V.T.; Hwang, S.S.; Feuerman, M. Validation of the Edmonton Symptom Assessment Scale. Cancer 2000, 88, 2164–2171. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998; Part viii; p. 104. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Botega, N.J.; Bio, M.R.; Zomignani, M.A.; Garcia, C., Jr.; Pereira, W.A. Mood disorders among inpatients in ambulatory and validation of the anxiety and depression scale HAD. Rev. Saude Publica 1995, 29, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Brorsson, B.; Asberg, K.H. Katz index of independence in ADL. Reliability and validity in short-term care. Scand. J. Rehabil. Med. 1984, 16, 125–132. [Google Scholar]
- Jung, T.; Moorhouse, N.; Shi, X.; Amin, M.F. A Virtual Reality-Supported Intervention for Pulmonary Rehabilitation of Patients with Chronic Obstructive Pulmonary Disease: Mixed Methods Study. J. Med. Internet Res. 2020, 22, e14178. [Google Scholar] [CrossRef]
- Gift, A.G.; Pugh, L.C. Dyspnea and fatigue. Nurs. Clin. N. Am. 1993, 28, 373–384. [Google Scholar]
- Marlow, L.L.; Faull, O.K.; Finnegan, S.L.; Pattinson, K.T.S. Breathlessness and the brain: The role of expectation. Curr. Opin. Support. Palliat. Care 2019, 13, 200–210. [Google Scholar] [CrossRef]
- Gibbons, C.H. Basics of autonomic nervous system function. Handb. Clin. Neurol. 2019, 160, 407–418. [Google Scholar] [CrossRef]
- Gullich, I.; Ramos, A.B.; Zan, T.R.; Scherer, C.; Mendoza-Sassi, R.A. Prevalence of anxiety in patients admitted to a university hospital in southern Brazil and associated factors. Rev. Bras. Epidemiol. 2013, 16, 644–657. [Google Scholar] [CrossRef] [Green Version]
- Rutkowski, S.; Szczegielniak, J.; Szczepanska-Gieracha, J. Evaluation of the Efficacy of Immersive Virtual Reality Therapy as a Method Supporting Pulmonary Rehabilitation: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 352. [Google Scholar] [CrossRef]
- Koepp, M.J.; Gunn, R.N.; Lawrence, A.D.; Cunningham, V.J.; Dagher, A.; Jones, T.; Brooks, D.J.; Bench, C.J.; Grasby, P.M. Evidence for striatal dopamine release during a video game. Nature 1998, 393, 266–268. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Experimental (n = 22) | Control (n = 22) | p-Value |
|---|---|---|---|
| Age (Mean ± SD) | 48.9 ± 13.9 | 48.5 ± 16.9 | 0.998 |
| Male gender n (%) | 11 (50%) | 11 (50%) | 1.00 |
| MMSE (Mean ± SD) | 24.9 ±4 | 24 ± 3.8 | 0.668 |
| Katz Index (Mean ± SD) | 2.5 ± 2 | 2.8 ± 2.5 | 0.736 |
| Variables | Experimental Group | Control Group | EG vs. CG | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post Intervention | p-Value | Effect Size | Baseline | Post Intervention | p-Value | Effect Size | Effect Size Post vs. Post | |
| ESRS | |||||||||
| Pain | 1.20 (±1.78) | 0.90 (±1.87) | 0.394 | 0.164523 | 0.88 (±1.81) | 0.59 (±1.24) | 0.414 | 0.189524 | 0.19666 |
| Weariness | 2.60 (±3.09) | 1.45 (±2.36) * | 0.005 | 0.418701 | 1.35 (±1.81) | 0.71 (±1.27) * | 0.026 | 0.413114 | 0.39309 |
| Somnolence | 1.25 (±2.05) | 0.95 (±2.27) | 0.340 | 0.138861 | 1.71 (±2.02) | 1.29 (±1.64) | 0.084 | 0.223836 | 0.173964 |
| Nausea | 0.05 (±0.22) | 0.00 (0.00) | 0.317 | - | 0.29 (±0.75) | 0.24 (±0.73) | 0.655 | 0.079555 | - |
| Appetite | 1.15 (±2.13) | 0.60 (±1.16) | 0.066 | 0.321108 | 1.00 (±2.40) | 0.65 (±1.97) | 0.180 | 0.16076 | 0.029141 |
| Shortness of breath | 1.70 (±2.70) | 1.15 (±2.15) * | 0.026 | 0.225124 | 1.53 (±3.01) | 0.65 (±1.53) | 0.078 | 0.369275 | 0.269344 |
| Depression | 1.90 (±2.72) | 1.90 (±2.88) | 0.394 | 0.0 | 2.71 (±3.01) | 2.35 (±2.76) | 0.336 | 0.122245 | 0.16049 |
| Anxiety | 4.10 (±2.90) | 2.10 (±2.23) * | 0.001 | 0.773245 | 4.18 (±3.20) | 2.94 (±2.55) * | 0.011 | 0.426358 | 0.350552 |
| Well-being | 3.65 (±2.50) | 2.30 (±2.79) * | 0.029 | 0.50957 | 4.53 (±3.03) | 4.18 (±2.87) | 0.523 | 0.119475 | 0.661925 # |
| BSPE | 12.13 (±4.55) | 10.63 (±3.07) | 0.063 | 0.386609 | 12.50 (±5.93) | 12.38 (±5.93) | 1.000 | 0.021089 | 0.370851 |
| HADS | 9.83 (±4.31) | 7.17 (±2.79) * | 0.042 | 0.734904 | 15.00 (±10.31) | 13.00 (±9.49) | 0.075 | 0.201883 | 0.000001 |
| Variables | Experimental Group | Control Group | EG vs. CG | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post Intervention | p-Value | Effect Size | Baseline | Post Intervention | p-Value | Effect Size | Effect Size Post vs. Post | |
| SBP (mmHg) | 122.37 (±14.44) | 119.32 (±13.76) | 0.178 | 0.216249 | 128.24 (±22.01) | 130.65 (±19.87) | 0.780 | 0.114941 | 0.66295 #a |
| DPB (mmHg) | 75.79 (±18.06) | 73.47 (±17.80) | 0.378 | 0.129389 | 74.12 (±28.24) | 67.53 (±25.40) | 0.056 | 0.245368 | 0.270841 |
| SpO2 (%) | 94.27 (±4.63) | 95.64 (±3.58) * | 0.016 | 0.331043 | 94.17 (±2.73) | 94.94 (±2.69) * | 0.018 | 0.284125 | 0.221069 |
| HR (BPM) | 86.95 (±15.34) | 84.76 (±17.59) | 0.080 | 0.1327 | 97.28 (±19.76) | 96.33 (±19.04) | 0.604 | 0.048961 | 0.631228 #b |
| RR (irpm) | 26.41 (±9.89) | 23.41 (±7.60) * | 0.015 | 0.34015 | 23.50 (±6.32) | 22.36 (±5.90) * | 0.037 | 0.186469 | 0.154337 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, I.M.; Lima, A.G.; Santos, A.E.d.; Santos, A.C.A.; Nascimento, L.S.d.; Serra, M.V.C.L.; Pereira, T.d.J.S.; Barbosa, F.D.S.; Seixas, V.M.; Monte-Silva, K.; et al. A Single Session of Virtual Reality Improved Tiredness, Shortness of Breath, Anxiety, Depression and Well-Being in Hospitalized Individuals with COVID-19: A Randomized Clinical Trial. J. Pers. Med. 2022, 12, 829. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12050829
Rodrigues IM, Lima AG, Santos AEd, Santos ACA, Nascimento LSd, Serra MVCL, Pereira TdJS, Barbosa FDS, Seixas VM, Monte-Silva K, et al. A Single Session of Virtual Reality Improved Tiredness, Shortness of Breath, Anxiety, Depression and Well-Being in Hospitalized Individuals with COVID-19: A Randomized Clinical Trial. Journal of Personalized Medicine. 2022; 12(5):829. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12050829
Chicago/Turabian StyleRodrigues, Isabele Moraes, Adriana Gomes Lima, Ana Evelyn dos Santos, Anne Carolline Almeida Santos, Luciana Silva do Nascimento, Maria Veronica Cavalcanti Lins Serra, Terezinha de Jesus Santos Pereira, Felipe Douglas Silva Barbosa, Valquiria Martins Seixas, Katia Monte-Silva, and et al. 2022. "A Single Session of Virtual Reality Improved Tiredness, Shortness of Breath, Anxiety, Depression and Well-Being in Hospitalized Individuals with COVID-19: A Randomized Clinical Trial" Journal of Personalized Medicine 12, no. 5: 829. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12050829