Gender-Specific Differences in the Intensive Care Treatment of COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohorts
2.2. Data Collection
2.3. Statistical Analysis
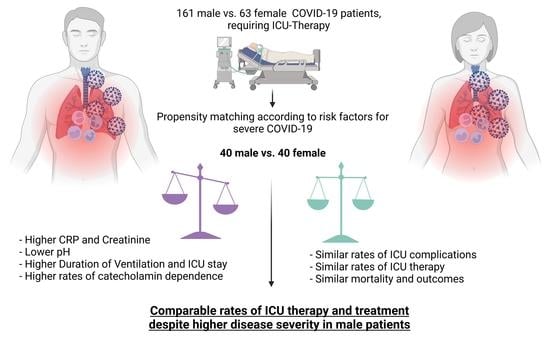
3. Results
3.1. Baseline Characteristics
3.2. Propensity Score Matching
3.3. Characteristics of Matched Groups-Input
3.4. Therapy at the ICU—Output
3.5. Complications—Outcome
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John Hopkins University. Johns Hopkins Coronavirus Resource Center. Available online: https://0-coronavirus-jhu-edu.brum.beds.ac.uk/map.html. (accessed on 23 March 2022).
- Giovanetti, M.; Benedetti, F.; Campisi, G.; Ciccozzi, A.; Fabris, S.; Ceccarelli, G.; Tambone, V.; Caruso, A.; Angeletti, S.; Zella, D.; et al. Evolution patterns of SARS-CoV-2: Snapshot on its genome variants. Biochem. Biophys. Res. Commun. 2021, 538, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Price, A.; Barlow-Pay, F.; Duffy, S.; Pearce, L.; Vilches-Moraga, A.; Moug, S.; Quinn, T.; Stechman, M.; Braude, P.; Mitchell, E.; et al. Study protocol for the COPE study: COVID-19 in Older PEople: The influence of frailty and multimorbidity on survival. A multicentre, European observational study. BMJ Open 2020, 10, 2020–040569. [Google Scholar] [CrossRef] [PubMed]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Pillay, T.S. Gene of the month: The 2019-nCoV/SARS-CoV-2 novel coronavirus spike protein. J. Clin. Pathol. 2020, 73, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, U.M.; Abokor, A.A.; Edwards, J.M.; Waigi, E.W.; Royfman, R.S.; Hasan, S.A.; Smedlund, K.B.; Hardy, A.M.G.; Chakravarti, R.; Koch, L.G. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol. Genom. 2021, 53, 51–60. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Niu, S. ACE2 and COVID-19 and the resulting ARDS. Postgrad. Med. J. 2020, 96, 403–407. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Lashgari, N.A.; Momeni Roudsari, N.; Momtaz, S.; Abdolghaffari, A.H. Transmembrane serine protease 2 and angiotensin-converting enzyme 2 anti-inflammatory receptors for COVID-19/inflammatory bowel diseases treatment. World J. Gastroenterol. 2021, 27, 7943–7955. [Google Scholar] [CrossRef]
- Seyed Hosseini, E.; Riahi Kashani, N.; Nikzad, H.; Azadbakht, J.; Hassani Bafrani, H.; Haddad Kashani, H. The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology 2020, 551, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef] [PubMed]
- Balk, R.A. Systemic inflammatory response syndrome (SIRS): Where did it come from and is it still relevant today? Virulence 2014, 5, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jirak, P. Herausforderungen in der Versorgung und Nachbetreuung Multimorbider Patienten und Patientinnen im Kontext Einer COVID-19-Erkrankung; Paracelsus Medizinische Privatuniversität: Salzburg, Austria, 2021. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Yanez, N.D.; Weiss, N.S.; Romand, J.A.; Treggiari, M.M. COVID-19 mortality risk for older men and women. BMC Public Health 2020, 20, 020–09826. [Google Scholar] [CrossRef]
- Mukherjee, S.; Pahan, K. Is COVID-19 Gender-sensitive? J. Neuroimmune Pharmacol. 2021, 16, 38–47. [Google Scholar] [CrossRef]
- Fish, E.N. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef]
- Babapoor-Farrokhran, S.; Gill, D.; Walker, J.; Rasekhi, R.T.; Bozorgnia, B.; Amanullah, A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci 2020, 253, 28. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, H. Versorgungsforschung–Begriffsbestimmung, Gegenstand und Aufgaben; Huber: Bern, Switzerland, 2003; pp. 13–23. [Google Scholar]
- Bienvenu, L.A.; Noonan, J.; Wang, X.; Peter, K. Higher mortality of COVID-19 in males: Sex differences in immune response and cardiovascular comorbidities. Cardiovasc. Res. 2020, 116, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Bleker, S.M.; Coppens, M.; Middeldorp, S. Sex, thrombosis and inherited thrombophilia. Blood Rev. 2014, 28, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Elhusseiny, K.M.; Yeh, Y.C.; Sun, W.Z. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-A systematic review and meta-analysis. PLoS ONE 2021, 16, e0246318. [Google Scholar] [CrossRef]
- Ciarambino, T.; Para, O.; Giordano, M. Immune system and COVID-19 by sex differences and age. Womens Health 2021, 17, 17455065211022262. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Hollinger, A.; Gayat, E.; Féliot, E.; Paugam-Burtz, C.; Fournier, M.C.; Duranteau, J.; Lefrant, J.Y.; Leone, M.; Jaber, S.; Mebazaa, A.; et al. Gender and survival of critically ill patients: Results from the FROG-ICU study. Ann. Intensive Care 2019, 9, 019–0514. [Google Scholar] [CrossRef] [Green Version]
- Lat, T.I.; McGraw, M.K.; White, H.D. Gender Differences in Critical Illness and Critical Care Research. Clin. Chest Med. 2021, 42, 543–555. [Google Scholar] [CrossRef]
- Madsen, T.E.; Napoli, A.M. The DISPARITY-II study: Delays to antibiotic administration in women with severe sepsis or septic shock. Acad. Emerg. Med. 2014, 21, 1499–1502. [Google Scholar] [CrossRef] [Green Version]
- Pietropaoli, A.P.; Glance, L.G.; Oakes, D.; Fisher, S.G. Gender differences in mortality in patients with severe sepsis or septic shock. Gend. Med. 2010, 7, 422–437. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Tong, L.; Yao, J.; Guo, Z.; Lui, K.Y.; Hu, X.; Cao, L.; Zhu, Y.; Huang, F.; Guan, X.; et al. Association of Sex With Clinical Outcome in Critically Ill Sepsis Patients: A Retrospective Analysis of the Large Clinical Database MIMIC-III. Shock 2019, 52, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Godeau, D.; Petit, A.; Richard, I.; Roquelaure, Y.; Descatha, A. Return-to-work, disabilities and occupational health in the age of COVID-19. Scand. J. Work Environ. Health 2021, 47, 408–409. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Olezene, C.S.; Hansen, E.; Steere, H.K.; Giacino, J.T.; Polich, G.R.; Borg-Stein, J.; Zafonte, R.D.; Schneider, J.C. Functional outcomes in the inpatient rehabilitation setting following severe COVID-19 infection. PLoS ONE 2021, 16, e0248824. [Google Scholar] [CrossRef] [PubMed]
- Gristina, G.R.; Piccinni, M. COVID-19 pandemic in ICU. Limited resources for many patients: Approaches and criteria for triaging. Minerva Anestesiol. 2021, 87, 1367–1379. [Google Scholar] [CrossRef]
- Yin, G.; Song, H.; Wang, J.; Nicholas, S.; Maitland, E. The COVID-19 Run on Medical Resources in Wuhan China: Causes, Consequences and Lessons. Healthcare 2021, 9, 1362. [Google Scholar] [CrossRef]
- Joebges, S.; Biller-Andorno, N. Ethics guidelines on COVID-19 triage-an emerging international consensus. Crit. Care 2020, 24, 201. [Google Scholar] [CrossRef]
- Williams, D.R. The health of men: Structured inequalities and opportunities. Am. J. Public Health 2003, 93, 724–731. [Google Scholar] [CrossRef]
- WHO. Tracking SARS-CoV-2 Variants; WHO: Geneva, Switzerland, 2021. [Google Scholar]


| Male (n = 161) | Female (n = 63) | p-Value | Std. Diff. | |||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | |||
| Age (years) | 68 | 65–79 | 64 | 58–78 | 0.212 | 0.27 |
| CHA2DS2-VASc score | 2 | 2–4 | 3 | 2–4 | 0.548 | 0.58 |
| BMI (kg/m2) | 27.7 | 25.3–30.4 | 28.5 | 25.3–35.1 | 0.315 | 0.13 |
| % | n | % | n | p-value | ||
| Coronary artery disease | 23.0 | 37 | 7.9 | 5 | 0.012 * | 0.33 |
| Diabetes mellitus | 33.5 | 54 | 34.9 | 22 | 0.876 | 0.01 |
| Arterial hypertension | 61.5 | 99 | 66.7 | 42 | 0.539 | 0.08 |
| History of smoking | 27.3 | 44 | 20.6 | 13 | 0.314 | 0.30 |
| Peripheral artery disease | 7.5 | 12 | 1.6 | 1 | 0.117 | 0.39 |
| Heart failure | 11.8 | 19 | 12.7 | 8 | 0.990 | 0.11 |
| Valvular heart disease | 8.1 | 13 | 6.3 | 4 | 0.785 | 0.15 |
| Obstructive pulmonary disease | 18.0 | 29 | 25.4 | 16 | 0.265 | 0.11 |
| Structural pulmonary disease | 5.6 | 9 | 11.1 | 7 | 0.247 | 0.07 |
| Pulmonary arterial hypertension | 3.1 | 5 | 3.2 | 2 | 0.990 | 0.11 |
| Atrial fibrillation | 15.5 | 25 | 15.9 | 10 | 0.949 | 0.03 |
| History of stroke | 6.2 | 10 | 6.3 | 4 | 0.969 | 0.15 |
| History of thrombosis | 6.2 | 10 | 15.9 | 10 | 0.023 * | 0.38 |
| Malignancy | 8.7 | 14 | 7.9 | 5 | 0.855 | 0.17 |
| Male (n = 40) | Female (n = 40) | p-Value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Age (years) | 64 | 58–78 | 68 | 65–79 | 0.212 |
| CHA2DS2-VASc score | 2 | 2–4 | 3 | 2–4 | 0.548 |
| BMI (kg/m2) | 27.7 | 25.3–30.4 | 28.5 | 25.3–35.1 | 0.315 |
| CK max (U/L) | 321 | 172–731 | 323 | 124–567 | 0.339 |
| CK-MB max (U/L) | 25 | 19–38 | 30 | 23–44 | 0.302 |
| High-sensitivity troponine max (% ULN) | 336 | 164–789 | 183 | 101–494 | 0.091 |
| pBNP max (pg/mL) | 976 | 355–4280 | 1189 | 546–4255 | 0.439 |
| Lactate max (U/L) | 2.67 | 2.12–4.00 | 3.05 | 2.18–4.74 | 0.617 |
| pH min | 7.20 | 7.13–7.32 | 7.31 | 7.15–7.40 | 0.014 * |
| Creatinine max (mg/dL) | 1.61 | 1.11–3.50 | 1.10 | 0.84–2.13 | 0.027 * |
| Potassium min (mmol/L) | 3.4 | 3.3–3.6 | 3.3 | 3.2–3.8 | 0.480 |
| Leukocyte count max (G/L) | 14.0 | 11.4–23.1 | 18.8 | 11.6–25.5 | 0.169 |
| Lymphocyte min (G/L) | 3.9 | 0.8–7.3 | 4.3 | 1.0–9.2 | 0.347 |
| CRP max (ng/mL) | 26.7 | 16.13–32.6 | 18.6 | 11.9–26.8 | 0.012 * |
| PCT max (ng/mL) | 1.00 | 0.59–2.90 | 0.69 | 0.22–1.97 | 0.152 |
| Interleukin 6 max (pg/mL) | 436 | 160–1347 | 161 | 61–545 | 0.020 * |
| Male (n = 40) | Female (n = 40) | p-Value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Duration of corticosteroids (days) | 8 | 0–10 | 9 | 0–10 | 0.879 |
| Stay on ICU (days) | 13 | 6–25 | 10 | 5–15 | 0.022 * |
| Time intubated (days) | 12 | 4–18 | 6 | 0–11 | 0.017 * |
| % | n | % | n | p-value | |
| Corticosteroids | 62.5 | 25 | 67.5 | 27 | 0.815 |
| Remdesivir | 10.0 | 4 | 17.5 | 7 | 0.518 |
| Tacilizumab | 15.0 | 6 | 7.7 | 3 | 0.481 |
| Therapeutic anticoagulation | 67.5 | 27 | 77.5 | 31 | 0.453 |
| Catecholamines | 87.2 | 34 | 67.5 | 27 | 0.037 * |
| Intubation | 85.0 | 34 | 75.0 | 30 | 0.402 |
| ECMO | 20.0 | 8 | 17.5 | 7 | 0.775 |
| Hemofiltration | 22.5 | 9 | 12.5 | 5 | 0.378 |
| Male (n = 40) | Female (n = 40) | p-Value | |||
|---|---|---|---|---|---|
| % | n | % | n | ||
| Death | 56.8 | 21 | 40.0 | 16 | 0.174 |
| Pulmonary embolism | 0.0 | 0 | 10.0 | 4 | 0.116 |
| Thrombosis | 5.0 | 2 | 5.0 | 2 | 0.990 |
| Stroke | 2.5 | 1 | 2.5 | 1 | 0.990 |
| Ventricular arrhythmias | 10.3 | 4 | 5.0 | 2 | 0.432 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jirak, P.; Mirna, M.; Van Almsick, V.; Shomanova, Z.; Mahringer, M.; Lichtenauer, M.; Kopp, K.; Topf, A.; Sieg, F.; Kraus, J.; et al. Gender-Specific Differences in the Intensive Care Treatment of COVID-19 Patients. J. Pers. Med. 2022, 12, 849. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12050849
Jirak P, Mirna M, Van Almsick V, Shomanova Z, Mahringer M, Lichtenauer M, Kopp K, Topf A, Sieg F, Kraus J, et al. Gender-Specific Differences in the Intensive Care Treatment of COVID-19 Patients. Journal of Personalized Medicine. 2022; 12(5):849. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12050849
Chicago/Turabian StyleJirak, Peter, Moritz Mirna, Vincent Van Almsick, Zornitsa Shomanova, Magdalena Mahringer, Michael Lichtenauer, Kristen Kopp, Albert Topf, Franz Sieg, Johannes Kraus, and et al. 2022. "Gender-Specific Differences in the Intensive Care Treatment of COVID-19 Patients" Journal of Personalized Medicine 12, no. 5: 849. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12050849