How Diet-Induced Changes in the “Gut-Liver” Axis Affect Chronic Liver Disease Outcome?
Abstract
:1. Introduction
2. Diet Has a Direct Effect on the Gut-Liver Axis
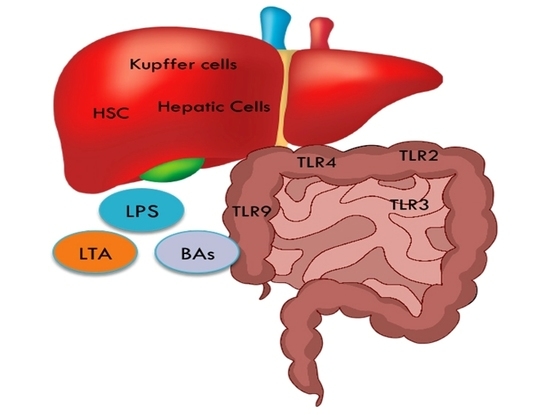
3. The Gut-Liver Axis
4. How Does A HCC Protective Diet Influence the Gut Microbiome?
5. Bacterial Components and Metabolites
5.1. LPS
5.2. LTA
5.3. Bile Acids
6. Epigenetic Control of Treg Cells through SCFAs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Giannitrapani, L.; Zerbo, M.; Amodeo, S.; Pipitone, E.; Galia, M.; Li Cavoli, T.V.; Minissale, M.G.; Licata, A.; Schiavone, C.; Brancatelli, G.; et al. The Changing Epidemiology of Hepatocellular Carcinoma: Experience of a Single Center. Biomed Res. Int. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- Asgharpour, A.; Cazanave, S.C.; Pacana, T.; Seneshaw, M.; Vincent, R.; Banini, B.A.; Kumar, D.P.; Daita, K.; Min, H.K.; Mirshahi, F.; et al. A Diet-Induced Animal Model of Non-Alcoholic Fatty Liver Disease and Hepatocellular Cancer. J. Hepatol. 2016, 65, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Koumbi, L. Dietary Factors Can Protect against Liver Cancer Development. World J. Hepatol. 2017, 9, 119–125. [Google Scholar] [CrossRef]
- Gorham, J.; Gleeson, M. Cirrhosis and Dysbiosis: New Insights from next-Generation Sequencing. Hepatology 2016, 63, 336–338. [Google Scholar] [CrossRef]
- Raj, A.S.; Shanahan, E.R.; Tran, C.D.; Bhat, P.; Fletcher, L.M.; Vesey, D.A.; Morrison, M.; Holtmann, G.; Macdonald, G.A. Dysbiosis of the Duodenal Mucosal Microbiota Is Associated with Increased Small Intestinal Permeability in Chronic Liver Disease. Clin. Transl. Gastroenterol. 2019, 10. [Google Scholar] [CrossRef]
- Dapito, D.H.; Mencin, A.; Gwak, G.Y.; Pradere, J.P.; Jang, M.K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of Hepatocellular Carcinoma by the Intestinal Microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Sui, J.; Ma, Y.; Simon, T.G.; Chong, D.; Meyerhardt, J.A.; Willett, W.C.; Giovannucci, E.L.; Chan, A.T.; Zhang, X. A Prospective Study of Dairy Product Intake and the Risk of Hepatocellular Carcinoma in U.S. Men and Women. Int. J. Cancer 2020, 146, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Tieri, M.; Ghelfi, F.; Titta, L.; Marventano, S.; Lafranconi, A.; Gambera, A.; Alonzo, E.; Sciacca, S.; Buscemi, S.; et al. Dairy Foods and Health: An Umbrella Review of Observational Studies. Int. J. Food Sci. Nutr. 2020, 71, 138–151. [Google Scholar] [CrossRef]
- Talamini, R.; Polesel, J.; Montella, M.; Dal Maso, L.; Crispo, A.; Tommasi, L.G.; Izzo, F.; Crovatto, M.; La Vecchia, C.; Franceschi, S. Food Groups and Risk of Hepatocellular Carcinoma: A Multicenter Case-Control Study in Italy. Int. J. Cancer 2006, 119, 2916–2921. [Google Scholar] [CrossRef] [PubMed]
- Tieri, M.; Ghelfi, F.; Vitale, M.; Vetrani, C.; Marventano, S.; Lafranconi, A.; Godos, J.; Titta, L.; Gambera, A.; Alonzo, E.; et al. Whole Grain Consumption and Human Health: An Umbrella Review of Observational Studies. Int. J. Food Sci. Nutr. 2020, 71, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, Y.; Liu, J.; Lu, K.; Tang, Z.; Liu, P.; Liu, L.; Zhu, Y. Systematic Review with Meta-Analysis: Meat Consumption and the Risk of Hepatocellular Carcinoma. Aliment. Pharmacol. Ther. 2014, 39, 913–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Godos, J.; Ghelfi, F.; Tieri, M.; Titta, L.; Lafranconi, A.; Marventano, S.; Alonzo, E.; Gambera, A.; Sciacca, S.; et al. Fruit and Vegetable Consumption and Health Outcomes: An Umbrella Review of Observational Studies. Int. J. Food Sci. Nutr. 2019, 70, 652–667. [Google Scholar] [CrossRef]
- Gao, M.; Sun, K.; Guo, M.; Gao, H.; Liu, K.; Yang, C.; Li, S.; Liu, N. Fish Consumption and N-3 Polyunsaturated Fatty Acids, and Risk of Hepatocellular Carcinoma: Systematic Review and Meta-Analysis. Cancer Causes Control 2015, 26, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Shawon, M.A.-A.; Yousuf, M.A.K.; Raheem, E.; Ahmed, S.; Dipti, T.T.; Hoque, M.R.; Taniguchi, H.; Karim, M.R. Epidemiology, Clinical Features, and Impact of Food Habits on the Risk of Hepatocellular Carcinoma: A Case-Control Study in Bangladesh. PLoS ONE 2020, 15, e0232121. [Google Scholar] [CrossRef]
- Marventano, S.; Godos, J.; Tieri, M.; Ghelfi, F.; Titta, L.; Lafranconi, A.; Gambera, A.; Alonzo, E.; Sciacca, S.; Buscemi, S.; et al. Egg Consumption and Human Health: An Umbrella Review of Observational Studies. Int. J. Food Sci. Nutr. 2020, 71, 325–331. [Google Scholar] [CrossRef]
- Fedirko, V.; Lukanova, A.; Bamia, C.; Trichopolou, A.; Trepo, E.; Nöthlings, U.; Schlesinger, S.; Aleksandrova, K.; Boffetta, P.; Tjønneland, A.; et al. Glycemic Index, Glycemic Load, Dietary Carbohydrate, and Dietary Fiber Intake and Risk of Liver and Biliary Tract Cancers in S. Ann. Oncol. 2013, 24, 543–553. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, W.; Li, T.; Liu, Y.; Simon, T.G.; Sui, J.; Wu, K.; Giovannucci, E.L.; Chan, A.T.; Zhang, X. Meat Intake and Risk of Hepatocellular Carcinoma in Two Large US Prospective Cohorts of Women and Men. Int. J. Epidemiol. 2019, 48, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Chern, H.D.; Wu, J.C.; Chao, Y.; Huang, Y.H.; Chang, F.Y.; Lee, S.D. Polymorphism of the N-Acetyltransferase 2 Gene, Red Meat Intake, and the Susceptibility of Hepatocellular Carcinoma. Am. J. Gastroenterol. 2003, 98, 1417–1422. [Google Scholar] [CrossRef]
- Fedirko, V.; Trichopolou, A.; Bamia, C.; Duarte-Salles, T.; Trepo, E.; Aleksandrova, K.; Nöthlings, U.; Lukanova, A.; Lagiou, P.; Boffetta, P.; et al. Consumption of Fish and Meats and Risk of Hepatocellular Carcinoma: The European Prospective Investigation into Cancer and Nutrition (EPIC). Ann. Oncol. 2013, 24, 2166–2173. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the Human Gut Microbiome in Liver Cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Huang, R.-X.; Duan, Y.-Y.; Hu, J.-A. Fish Intake and Risk of Liver Cancer: A Meta-Analysis. PLoS ONE 2015, 10, e0096102. [Google Scholar] [CrossRef]
- Freedman, N.D.; Cross, A.J.; McGlynn, K.A.; Abnet, C.C.; Park, Y.; Hollenbeck, A.R.; Schatzkin, A.; Everhart, J.E.; Sinha, R. Association of Meat and Fat Intake with Liver Disease and Hepatocellular Carcinoma in the NIH-AARP Cohort. J. Natl. Cancer Inst. 2010, 102, 1354–1365. [Google Scholar] [CrossRef] [Green Version]
- George, E.S.; Sood, S.; Broughton, A.; Cogan, G.; Hickey, M.; Chan, W.S.; Sudan, S.; Nicoll, A.J. The Association between Diet and Hepatocellular Carcinoma: A Systematic Review. Nutrients 2021, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Endres, S.; Ghorbani, R.; Kelley, V.E.; Georgilis, K.; Lonnemann, G.; van der Meer, J.W.M.; Cannon, J.G.; Rogers, T.S.; Klempner, M.S.; Weber, P.C.; et al. The Effect of Dietary Supplementation with n—3 Polyunsaturated Fatty Acids on the Synthesis of Interleukin-1 and Tumor Necrosis Factor by Mononuclear Cells. N. Engl. J. Med. 1989, 320, 265–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negri, E.; La Vecchia, C.; Franceschi, S.; D’Avanzo, B.; Parazzini, F. Vegetable and Fruit Consumption and Cancer Risk. Int. J. Cancer 1991, 48, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Romieu, I.; Scalbert, A.; Slimani, N.; Hjartåker, A.; Engeset, D.; Skeie, G.; Overvad, K.; et al. Differences in Dietary Intakes, Food Sources and Determinants of Total Flavonoids between Mediterranean and Non-Mediterranean Countries Participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Br. J. Nutr. 2013, 109, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Daniell, E.L.; Ryan, E.P.; Brick, M.A.; Thompson, H.J. Dietary Dry Bean Effects on Hepatic Expression of Stress and Toxicity-Related Genes in Rats. Br. J. Nutr. 2020, 108, S37–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtani, N.; Kawada, N. Role of the Gut-Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship. Hepatol. Commun. 2019, 3, 456–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiest, R.; Albillos, A.; Trauner, M.; Bajaj, J.S.; Jalan, R. Targeting the Gut-Liver Axis in Liver Disease. J. Hepatol. 2017, 67, 1084–1103. [Google Scholar] [CrossRef] [Green Version]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, M.A.; Holscher, H.D. Microbiome-Mediated Effects of the Mediterranean Diet on Inflammation. Adv. Nutr. 2018, 9, 93–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Luedde, T. Apoptosis and Necroptosis in the Liver: A Matter of Life and Death. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; De Minicis, S.; Österreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 Enhances TGF-β Signaling and Hepatic Fibrosis. Nat. Med. 2007, 13, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.M.; Kamachi, F.; Watanabe, Y.; Yoshimoto, S.; Kanda, H.; Arai, Y.; Nakajima-Takagi, Y.; Iwama, A.; Koga, T.; Sugimoto, Y.; et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through Pge2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017, 7, 522–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-Induced Gut Microbial Metabolite Promotes Liver Cancer through Senescence Secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Imajo, K.; Fujita, K.; Yoneda, M.; Nozaki, Y.; Ogawa, Y.; Shinohara, Y.; Kato, S.; Mawatari, H.; Shibata, W.; Kitani, H.; et al. Hyperresponsivity to Low-Dose Endotoxin during Progression to Nonalcoholic Steatohepatitis Is Regulated by Leptin-Mediated Signaling. Cell Metab. 2012, 16, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Eliopoulos, A.G.; Dumitru, C.D.; Wang, C.C.; Cho, J.; Tsichlis, P.N. Induction of COX-2 by LPS in Macrophages Is Regulated by Tpl2-Dependent CREB Activation Signals. EMBO J. 2002, 21, 4831–4840. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Takashina, Y.; Watanabe, M.; Nagamine, R.; Saito, Y.; Kamada, N.; Saito, H. Bile Acid Metabolism Regulated by the Gut Microbiota Promotes Non-Alcoholic Steatohepatitis-Associated Hepatocellular Carcinoma in Mice. Oncotarget 2018, 9, 9925–9939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut Microbiome–Mediated Bile Acid Metabolism Regulates Liver Cancer via NKT Cells. Science 2018, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Worsley, O.; Yang, E.; Purbojati, R.W.; Liang, A.L.; Tan, W.; Moses, D.I.D.; Hartono, S.; Fan, V.; Lim, T.K.H.; et al. Persistent Changes in Liver Methylation and Microbiome Composition Following Reversal of Diet-Induced Non-Alcoholic-Fatty Liver Disease. Cell. Mol. Life Sci. 2019, 76, 4341–4354. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]

| Dietary Food Intake Associated with HCC Risk | |||
|---|---|---|---|
| Dietary Components | Association Type with HCC Risk | Country of Study | References |
| Dietary Fiber | Positive | Europe Rat Model | Fedirko et al. 2013 [17] Daniel et al. 2020 [18] |
| Fish | Inverse | Meta-analysis Meta-analysis USA Western Europe | Gao et al. 2015 [14] Huang et al. 2015 [19] Freedman et al. 2010 [20] Fedirko et al. 2013 [17] |
| Fruits | Inverse | Meta-analysis Europe | Schwingshackl et al. 2017 [12] Zamora-Ros et al. 2013 [21] |
| Glycemic load | Positive | Europe | Fedriko et al. 2013 [17] |
| Dairy products | Inverse | Meta-analysis USA | Godos et al. 2020 [8] Yang et al. 2020 [7] |
| Red meat | Positive | USA Europe | Ma et al. 2019 [22] Fedriko et al. 2013 [17] |
| White Meat | Inverse | Meta-analyses Western Europe | Luo et al. 2014 [11] Fedirko et al. 2013 [17] |
| Tea | Inverse | Italy | Talamini et al. 2006 [23] |
| Vegetables | Inverse | Bangladesh | Shawon et al. 2020 [15] Zamora-Ros et al. 2013 [21] |
| Toll Like Receptors | Cellular Targets in the Liver |
|---|---|
| TLR2, TLR4 and TLR9 | Hepatic stellate cells (HSC) |
| TLR2, TLR3, TLR4 and TLR9 | Kupffer cells |
| TLR1-9 | Hepatic cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koumbi, L.; Eliopoulos, A.G.; Vassilopoulou, E. How Diet-Induced Changes in the “Gut-Liver” Axis Affect Chronic Liver Disease Outcome? Livers 2021, 1, 40-48. https://0-doi-org.brum.beds.ac.uk/10.3390/livers1010004
Koumbi L, Eliopoulos AG, Vassilopoulou E. How Diet-Induced Changes in the “Gut-Liver” Axis Affect Chronic Liver Disease Outcome? Livers. 2021; 1(1):40-48. https://0-doi-org.brum.beds.ac.uk/10.3390/livers1010004
Chicago/Turabian StyleKoumbi, Lemonica, Aristides G. Eliopoulos, and Emilia Vassilopoulou. 2021. "How Diet-Induced Changes in the “Gut-Liver” Axis Affect Chronic Liver Disease Outcome?" Livers 1, no. 1: 40-48. https://0-doi-org.brum.beds.ac.uk/10.3390/livers1010004