Study on Stability of Mechanical Properties for Porous Fe-Cr-Al Alloys after Long-Term Aging
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of the Synthesized Materials
3.2. Evolution of the Material Properties during Aging
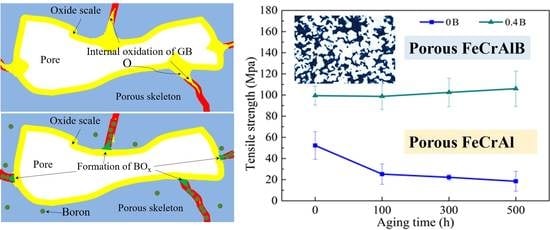
3.3. Mechanism for Mechanical Performance Degradation
3.3.1. The Influence of Phase Separation
3.3.2. The Influence of Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuomi, S.; Kurkela, E.; Simell, P.; Reinikainen, M. Behaviour of tars on the filter in high temperature filtration of biomass-based gasification gas. Fuel 2015, 139, 220–231. [Google Scholar] [CrossRef]
- Pauletto, G.; Vaccari, A.; Groppi, G.; Bricaud, L.; Benito, P.; Boffito, D.C.; Lercher, J.A.; Patience, G.S. FeCrAl as a Catalyst Support. Chem. Rev. 2020, 120, 7516–7550. [Google Scholar] [CrossRef] [PubMed]
- Molin, S.; Kusz, B.; Gazda, M.; Jasinski, P. Evaluation of porous 430L stainless steel for SOFC operation at intermediate temperatures. J. Power Sources 2008, 181, 31–37. [Google Scholar] [CrossRef]
- Molin, S.; Gazda, M.; Kusz, B.; Jasinski, P. Evaluation of 316L porous stainless steel for SOFC support. J. Eur. Ceram. Soc. 2009, 29, 757–762. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Wang, H.; Wang, X.; An, X.; He, K. Effects of Cr element on the crystal structure, microstructure, and mechanical properties of FeCrAl alloys. Mater. Sci. Eng. A 2021, 826, 142003. [Google Scholar] [CrossRef]
- Pint, B.A.; Terrani, K.A.; Brady, M.P.; Cheng, T.; Keiser, J.R. High temperature oxidation of fuel cladding candidate materials in steam–hydrogen environments. J. Nucl. Mater. 2013, 440, 420–427. [Google Scholar] [CrossRef]
- Engkvist, J.; Canovic, S.; Liu, F.; Götlind, H.; Svensson, J.-E.; Johansson, L.-G.; Olsson, M.; Halvarsson, M. Oxidation of FeCrAl foils at 500–900 °C in dry O2and O2with 40% H2O. Mater. High Temp. 2009, 26, 199–210. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, R.; Wang, H.; Xu, Y.; Wang, H. In Steam Short-Time Oxidation Kinetics of FeCrAl Alloys. J. Mater. Eng. Perform. 2018, 27, 6407–6414. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, H.; Liu, X.; Yu, H.; Wang, L.; Jiang, Y.; Gao, L.; Yu, L.; He, Y.; Chen, X.; et al. Reactive synthesis and assessment of porous Fe-20.5Al-18Cr intermetallic material: A comparative study with porous FeCrAl material produced from prealloyed powders. Sep. Purif. Technol. 2019, 220, 152–161. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Jiang, Y.; Gao, L.; Yu, L.; Lin, N.; He, Y.; Liu, C. Direct separation of arsenic and antimony oxides by high-temperature filtration with porous FeAl intermetallic. J. Hazard. Mater. 2017, 338, 364–371. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, D.; Yang, W.; Liu, L.; Guo, S.; Wang, S. Incipient corrosion of FeCrAl alloys in H3BO3- and LiOH-containing pure water at 360 °C and 18.5 MPa. J. Nucl. Mater. 2021, 557, 153299. [Google Scholar] [CrossRef]
- Maji, B.C.; Ukai, S.; Oono-Hori, N. Microstructural stability and intermetallic embrittlement in high Al containing FeCrAl-ODS alloys. Mater. Sci. Eng. A 2021, 807, 140858. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, H.; Yu, H.; Wang, L.; Liu, X.; Ma, J.; Zhang, N.; He, Y.; Jiang, Y. The role of Cr in the reactive synthesis of porous FeAlCr intermetallic compounds. Mater. Chem. Phys. 2020, 249, 123013. [Google Scholar] [CrossRef]
- Gussev, M.N.; Field, K.G.; Yamamoto, Y. Design, properties, and weldability of advanced oxidation-resistant FeCrAl alloys. Mater. Des. 2017, 129, 227–238. [Google Scholar] [CrossRef]
- Tang, C.; Jianu, A.; Steinbrueck, M.; Grosse, M.; Weisenburger, A.; Seifert, H.J. Influence of composition and heating schedules on compatibility of FeCrAl alloys with high-temperature steam. J. Nucl. Mater. 2018, 511, 496–507. [Google Scholar] [CrossRef] [Green Version]
- Unocic, K.A.; Yamamoto, Y.; Pint, B.A. Effect of Al and Cr Content on Air and Steam Oxidation of FeCrAl Alloys and Commercial APMT Alloy. Oxid. Met. 2017, 87, 431–441. [Google Scholar] [CrossRef]
- Wagner, C. Passivity and inhibition during the oxidation of metals at elevated temperatures. Corros. Sci. 1965, 5, 751–764. [Google Scholar] [CrossRef]
- Chevalier, S.; Bonnet, G.; Larpin, J.P.; Colson, J.C. The combined effect of refractory coatings containing reactive elements on high temperature oxidation behavior of chromia-forming alloys. Corros. Sci. 2003, 45, 1661–1673. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takasugi, T. Mapping of 475 °C embrittlement in ferritic Fe–Cr–Al alloys. Scr. Mater. 2010, 63, 1104–1107. [Google Scholar] [CrossRef]
- Chandra, D.; Schwartz, L.H. Mössbauer effect study of the 475°C decomposition of Fe-Cr. Met. Mater. Trans. A 1971, 2, 511–519. [Google Scholar] [CrossRef]
- Briggs, S.A.; Edmondson, P.D.; Littrell, K.C.; Yamamoto, Y.; Howard, R.H.; Daily, C.R.; Terrani, K.A.; Sridharan, K.; Field, K.G. A combined APT and SANS investigation of α′ phase precipitation in neutron-irradiated model FeCrAl alloys. Acta Mater. 2017, 129, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Ejenstam, J.; Thuvander, M.; Olsson, P.; Rave, F.; Szakalos, P. Microstructural stability of Fe–Cr–Al alloys at 450–550 °C. J. Nucl. Mater. 2015, 457, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wang, Z.X.; Xia, C.H.; Ouyang, M.H.; Peng, J.C.; Zhang, H.W.; Xiao, X.S. Aluminum suppression of α′ precipitate in model Fe–Cr–Al alloys during long-term aging at 475 °C. Mater. Sci. Eng. A 2019, 772, 138714. [Google Scholar] [CrossRef]
- Li, W.; Lu, S.; Hu, Q.-M.; Mao, H.; Johansson, B.; Vitos, L. The effect of Al on the 475 °C embrittlement of Fe–Cr alloys. Comput. Mater. Sci. 2013, 74, 101–106. [Google Scholar] [CrossRef]
- Hamana, D.; Amiour, L.; Bouchear, M. Effect of chromium ternary additions on the ordering behaviour in Fe–28at.% Al alloy. Mater. Chem. Phys. 2008, 112, 816–822. [Google Scholar] [CrossRef]
- Brady, M.P.; Tortorelli, P.F.; More, K.L.; Walker, L.R. Sulfidation–Oxidation Behavior of FeCrAl and TiCrAl and the Third-Element Effect. Oxid. Met. 2010, 74, 1–9. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, P.; Qi, W.; Du, S.; Liu, Z.; Meng, F.; Zhang, X.; Wang, K.; Li, Q.; Yao, Z.; et al. Mechanism of Al on FeCrAl steam oxidation behavior and molecular dynamics simulations. J. Alloy. Compd. 2020, 828, 154310. [Google Scholar] [CrossRef]
- Glasscock, J.A.; Mikkelsen, L.; Persson, H.; Pećanac, G.; Malzbender, J.; Blennow, P.; Bozza, F.; Hendriksen, P.V. Porous Fe21Cr7Al1Mo0.5Y metal supports for oxygen transport membranes: Thermo-mechanical properties, sintering and corrosion behaviour. Solid State Ionics 2013, 242, 33–44. [Google Scholar] [CrossRef]
- Schreiber, D.K.; Olszta, M.J.; Bruemmer, S.M. Directly correlated transmission electron microscopy and atom probe tomography of grain boundary oxidation in a Ni–Al binary alloy exposed to high-temperature water. Scr. Mater. 2013, 69, 509–512. [Google Scholar] [CrossRef]
- McCarroll, I.E.; La Fontaine, A.; Nguyen, T.D.; Smith, A.F.; Zhang, J.; Young, D.J.; Cairney, J.M. Performance of an FeCrAl alloy in a high-temperature CO2 environment. Corros. Sci. 2018, 139, 267–274. [Google Scholar] [CrossRef]
- Zhu, J.; Diaz, L.M.F.; Holcomb, G.R.; Jablonski, P.D.; Cowen, C.J.; Laughlin, D.E.; Alman, D.; Sridhar, S. On the Relation Between Oxide Ridge Evolution and Alloy Surface Grain Boundary Disorientation in Fe–22 wt % Cr Alloys. J. Electrochem. Soc. 2010, 157, B655. [Google Scholar] [CrossRef]
- Chan, K.S. A Grain Boundary Fracture Model for Predicting Dynamic Embrittlement and Oxidation-Induced Cracking in Superalloys. Met. Mater. Trans. A 2015, 46, 2491–2505. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Ma, J.; Yu, H.; Xu, S.; Hou, G.; He, Y.; Zheng, G. Reactive synthesis of porous FeAlCr intermetallics with enhanced mechanical property and oxidation resistance by introducing yttrium borides. Mater. Chem. Phys. 2021, 273, 124929. [Google Scholar] [CrossRef]
- Gao, H.; Xie, W.; Zhang, H.; Shen, W.; He, Y. Modification of the reactive synthesis of porous FeAl with addition of Si. Mater. High Temp. 2018, 36, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, W.; Gao, H.; Shen, W.; He, Y. Suppression of the SHS reactions during synthesis of porous FeAl intermetallics by introducing silicon. J. Alloy. Compd. 2018, 735, 1435–1438. [Google Scholar] [CrossRef]
- Gao, H.Y.; He, Y.H.; Zou, J.; Shen, P.Z.; Jiang, Y.; Liu, C.T. Mechanical properties of porous Fe–Al intermetallics. Powder Met. 2014, 58, 197–201. [Google Scholar] [CrossRef]
- Kondo, S.-I.; Masusaki, A.; Ogawa, K.; Morimura, T.; Nakashima, H. Effects of initial states on the spinodal decomposition of quenched and melt-spun Cu-15Ni-8Sn alloy. Mater. Trans. 2015, 56, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Field, K.G.; Littrell, K.C.; Briggs, S.A. Precipitation of α′ in neutron irradiated commercial FeCrAl alloys. Scr. Mater. 2018, 142, 41–45. [Google Scholar] [CrossRef]
- Capdevila, C.; Miller, M.K.; Toda, I.; Chao, J. Influence of the α–α′ phase separation on the tensile properties of Fe-base ODS PM 2000 alloy. Mater. Sci. Eng. A 2010, 527, 7931–7938. [Google Scholar] [CrossRef]
- Weisenburger, A.; Jianu, A.; Doyle, S.; Bruns, M.; Fetzer, R.; Heinzel, A.; DelGiacco, M.; An, W.; Müller, G. Oxide scales formed on Fe–Cr–Al-based model alloys exposed to oxygen containing molten lead. J. Nucl. Mater. 2013, 437, 282–292. [Google Scholar] [CrossRef]
- Mollard, M.; Rannou, B.; Bouchaud, B.; Balmain, J.; Bonnet, G.; Pedraza, F. Comparative degradation of nickel aluminized by slurry and by pack cementation under isothermal conditions. Corros. Sci. 2013, 66, 118–124. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; He, L.; Li, Z.; Yan, H.; Yang, T.; Ma, Y.; Peng, F.; Wu, Z. Oxidation behavior of (NbTaZrW)C high-entropy carbide at 800–1000 °C. Mater. Charact. 2022, 189, 111932. [Google Scholar] [CrossRef]
- Liu, C.; George, E.P. Environmental embrittlement in boron-free and boron-doped FeAl (40 at. % Al) alloys. Scr. Met. et Mater. 1990, 24, 1285–1290. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.-S.; Li, Y.-X.; Zhang, H.-W.; Liu, Y. Effects of carbon content on high-temperature mechanical and thermal fatigue properties of high-boron austenitic steels. China Foundry 2016, 13, 1–8. [Google Scholar] [CrossRef] [Green Version]










| Element (wt.%) | Fe20Cr5Al | Fe10Cr10Al | Fe10Cr20Al |
|---|---|---|---|
| Fe | 75 | 80 | 70 |
| Cr | 20 | 10 | 10 |
| Al | 5 | 10 | 20 |
| Samples | Porosity% | Tensile Strength (MPa) | Strain% | Hardness (HV) |
|---|---|---|---|---|
| Fe20Cr5Al | 33.1 | 52.3 | 6.9 | 127.3 |
| Fe10Cr10Al | 34.2 | 44.9 | 5.9 | 172.1 |
| Fe10Cr20Al | 39.4 | 35.1 | 2.3 | 261.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Ma, J.; Gao, Z.; Guo, F.; Xu, S.; Hou, G.; Zheng, G. Study on Stability of Mechanical Properties for Porous Fe-Cr-Al Alloys after Long-Term Aging. Materials 2022, 15, 3718. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15103718
Zhang H, Ma J, Gao Z, Guo F, Xu S, Hou G, Zheng G. Study on Stability of Mechanical Properties for Porous Fe-Cr-Al Alloys after Long-Term Aging. Materials. 2022; 15(10):3718. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15103718
Chicago/Turabian StyleZhang, Huibin, Junliang Ma, Zhencheng Gao, Fei Guo, Shenghang Xu, Guangya Hou, and Guoqu Zheng. 2022. "Study on Stability of Mechanical Properties for Porous Fe-Cr-Al Alloys after Long-Term Aging" Materials 15, no. 10: 3718. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15103718