Insights into Biodegradable Polymer-Supported Titanium Dioxide Photocatalysts for Environmental Remediation
Abstract
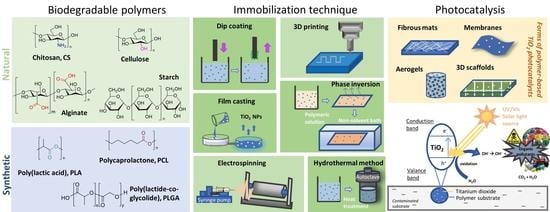
:1. Introduction
2. Biodegradable Polymers Combined with TiO2 for Enhanced Photocatalytic Activity
2.1. Natural Biodegradable Polymers
2.1.1. Chitosan (CS)
Synthetic and Characterization Routes
| Biodegradable Polymeric Matrix | Photocatalysis Parameters. | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Polymer Substrate | TiO2 Precursor | Dopant | Immobilization Technique | Morphology of the Photocatalyst | Type of (Target) Pollutant | Light Source | Degradation Efficiency (Time Required) | |
| 1 | CS | TiO2 nanopowders (Aeroxide; 80% anatase) | MT | CS-MT film casting & dip-coating in TiO2 formulation | Bilayer photocatalyst | Μethyl orange dye | 45 W fluorescent lamp | 98.7% | [23] |
| 2 | CS-grafted poly(vinyl imidazole) | Titanium isopropoxide | CDs | In situ deposition of TiO2 NPs and CDs onto the polymeric surface under microwave irradiation | Nanocomposite hydrogel | 2,4-dicholorophenol Reactive Blue 4 Reactive Red 15 | Sunlight exposure for 30 min | 95% (180 min) 95.8% (30 min) 98.2% (30 min) | [20] |
| 3 | CS | TiO2 (P25) | - | Immobilization of TiO2 in CS film by cross-linking process | Film | Tetracycline hydrochloride | UV lamp 30 W and λ = 360 nm | 87% (240 min) | [15] |
| 4 | CS | Aeroxide P25-TiO2 | - | 3D printing | 3D printed scaffolds | Amoxicillin | UV irradiation (125 W), λ = 300–800 nm | 90–60% (180 min) | [14] |
| 5 | CS | TiO2 powder (P25) | - | One-step spray-drying synthesis | CS/TiO2 nanocomposite particles | Organic dye, crystal violet | RPR-200 Photochemical Reactor (Rayonet), λ = 300 nm (8×, 21 W), and λ = 350 nm (8×, 24 W) lamps | 58.3–15.5% (120 min) 95.7% pristine particles | [25] |
| 6 | CS | TiO2 | GO | Dopped-GO and CS impregnated in TiO2 solution | - | cefixime trihydrate | 4 × lamps UV-A irradiation, λ = 365 nm | 95.34% (60 min) | [21] |
| 7 | CMCS | Butyl titanate | TiO2/ZrO2 composites | ZrO2:TiO2 were synthesized by a microwave hydrothermal method, CMCS as template | Composites | Rhodamine B | Photochemical reactor-UV irradiation (CEL-LPH120), | 90.5–60.6% (60 min) | [18] |
| 8 | CS + PVA | TiO2 (anatase) | Ag | Loading algae cells on the TiO2/Ag CS hybrid nanofiber mat prepared by electrospinning | Algae-TiO2/Ag hybrid nanofiber membrane | Cr(VI) removal | 500 W halogen tungsten lamp, λ > 400 nm | 91–25% (180 min) | [24] |
| 9 | CS + CA | TiO2 nanoparticles | SWCNTs + Fe3O4 | Incorporated inorganics into electrospun nanofibers | Composite nanofibers | Cr(VI), As(V), Methylene blue and Congo red | 4 × UV lamps, 30 W and λ = 365 nm | ~99% (40–60 min) | [22] |
Photocatalytic Performance
2.1.2. Cellulose
Synthetic and Characterization Routes
| Biodegradable Polymeric Matrix | Photocatalysis Parameters | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Polymer Substrate | TiO2 Precursor | Dopant | Immobilization Technique | Morphology of the Photocatalyst | Type of (Target) pollutant | Light Source | Degradation Efficiency (Time Required) | |
| 1 | Cellulose nanofibrils derived carbon + BC | Titanium isopropoxide (Ti(O-iPr)4, 99%) | - | One-pot hydrothermal method | Composite film and aerogel | Cr(VI) Rhodamine B (RhB) | Xenon lamp 300 W (CEL-HXF300), λ = 420 nm, visible light | 100% (60 min) 100% (60 min) | [10] |
| 2 | Cellulose | TiO2 (anatase) | Au nanoparticles | - Tape casting for the cellulose membrane - Simple suction filtration for the immobilization of Au and TiO2 NPs | Composite membranes | Rhodamine B (RhB) | 500 W Xe light simulation of sunlight | ~90% (180 min) | [26] |
| 3 | Cellulose | TiCl4 | Cu | Hydrolysis-precipitation method | Nanofibers | Organic dyes (reactive brilliant red K-2BP and cationic red X-GRL) | UV light, high pressure mercury lamp 300 w, λ = 365 nm | 96.57% (120 min) 99.73% (120 min) | [32] |
| 4 | Cellulose, cellulose triacetate | TiO2 nanoparticles | GO | Phase inversion via immersion precipitation method | Membranes | Benzene toluene, ethylbenzene xylenes (BTEX) | UVC light–UV lamp 100 W, λ = 280 nm | ~80% (180 min) ~70% (180 min) ~90% (180 min) ~75% (180 min) | [28] |
| 5 | Cellulose | Titanium n-butoxide solution | Ag2O nanoparticles | - Titania/cellulose composite by a surface sol–gel method - precipitation method for the nanocomposites | Hierarchical nanocomposites | Methylene Blue Rhodamine B Norfloxacin | UV light lamp 300 W | >90% (10 min) >90% (10 min) ~75% (10 min) | [29] |
| 6 | CMC | Commercial TiO2-Ag nanopowder | Ag nanoparticles | Film casting of CMC with gelatin, glycerol | Composite film | NH3 Ethanol Benzene | A closed system emmits light with λ = 254, 365, and 500 nm light | - - - | [4] |
| 7 | Cellulose | TiO2 Aeroxide P90 nanoparticles | Fe3O4 nanoparticles | Dropping cum phase separation for the nanocomposite spheres | Magnetic cellulose macrospheres | Cu2+ ions Rhodamine B | 2 × 15 W generates UV light λ = 365 nm | - 33-23% (60 min) | [31] |
| 8 | CAM | TiO2 P25 paste | - | Dip coating method | 2D and 3D TiO2 thin polymeric coated films | Cyanotoxins | Solar radiation simulator with a 1700 W air-cooled xenon arc lamp | 90% (12 kJ L−1) | [9] |
| 9 | Cellulose phosphate | TiO2 | - | Layer-by-layer (LbL) assembly technique | Porous multilayer films | Stearic acid Crystal violet Methylene Blue | UV radiation (16S Solar Light Simulator with a 150 W lamp | 95% (12 min) 95% (30 min) 50% (30 min) | [30] |
| 10 | Regenerated cellulose from recycled newspaper | Titanium-n-butoxide Ti(OBu)4 | - | Phase inversion method | Nano-composite membrane | Phenol degradation | 30 W UV lamp (λ = 312 nm), visible light from a 30 W-LED λ > 420 nm | 96% in UV light (360 min), 78.8% in visible light (360 min) | [27] |
Photocatalytic Performance
2.1.3. Alginate (Alg)
Synthetic and Characterization Routes
Photocatalytic Performance
2.1.4. Starch
Synthetic and Characterization Routes
Photocatalytic Performance
2.2. Synthetic Polymers with Biodegradable Nature
2.2.1. Poly(Lactic Acid) (PLA)
Synthetic and Characterization Routes
Photocatalytic Performance
2.2.2. Polycaprolactone (PCL)
Synthetic and Characterization Routes
Photocatalytic Performance
2.2.3. Other Synthetic Polymers
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malesic-Eleftheriadou, N.; Evgenidou, E.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of antibiotics in aqueous media by using new synthesized bio-based poly(ethylene terephthalate)-TiO2 photocatalysts. Chemosphere 2019, 234, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mahalingam, H.; Singh, P.K. Polymer-supported titanium dioxide photocatalysts for environmental remediation: A review. Appl. Catal. A Gen. 2013, 462–463, 178–195. [Google Scholar] [CrossRef]
- Xie, J.; Hung, Y.C. UV-A activated TiO2 embedded biodegradable polymer film for antimicrobial food packaging application. Lwt 2018, 96, 307–314. [Google Scholar] [CrossRef]
- Farshchi, E.; Pirsa, S.; Roufegarinejad, L.; Alizadeh, M.; Rezazad, M. Photocatalytic/biodegradable film based on carboxymethyl cellulose, modified by gelatin and TiO2-Ag nanoparticles. Carbohydr. Polym. 2019, 216, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Sökmen, M.; Tatlidil, I.; Breen, C.; Clegg, F.; Buruk, C.K.; Sivlim, T.; Akkan, Ş. A new nano-TiO2 immobilized biodegradable polymer with self-cleaning properties. J. Hazard. Mater. 2011, 187, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Piscitelli, F.; Buonocore, G.G.; Lavorgna, M.; Amendola, E.; Ambrosio, L. Effect of surface fluorination of TiO2 particles on photocatalitytic activity of a hybrid multilayer coating obtained by sol-gel method. ACS Appl. Mater. Interfaces 2012, 4, 150–157. [Google Scholar] [CrossRef]
- Kreetachat, T.; Kruenate, J.; Suwannahong, K. Preparation of TiO2/Bio-composite film by sol-gel method in VOCs photocatalytic degradation process. Appl. Mech. Mater. 2013, 390, 552–556. [Google Scholar] [CrossRef]
- Khodadadi, B. Facile sol–gel synthesis of Nd, Ce-codoped TiO2 nanoparticle using starch as a green modifier: Structural, optical and photocatalytic behaviors. J. Sol-Gel Sci. Technol. 2016, 80, 793–801. [Google Scholar] [CrossRef]
- Pinho, L.X.; Azevedo, J.; Miranda, S.M.; Ângelo, J.; Mendes, A.; Vilar, V.J.P.; Vasconcelos, V.; Boaventura, R.A.R. Oxidation of microcystin-LR and cylindrospermopsin by heterogeneous photocatalysis using a tubular photoreactor packed with different TiO2 coated supports. Chem. Eng. J. 2015, 266, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Qiu, J.; Xu, J.; Yao, J. Cellulose/TiO2-Based Carbonaceous Composite Film and Aerogel for Highly Efficient Photocatalysis under Visible Light. Ind. Eng. Chem. Res. 2020, 59, 13997–14003. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.U.; Ahn, K.; Kim, J.P.; Jin, J.S.; Lee, J.; Jeong, S.Y.; Cho, C.R. Enhanced photocatalytic activity of TiO2-incorporated nanofiber membrane by oxygen plasma treatment. Thin Solid Films 2011, 519, 6899–6902. [Google Scholar] [CrossRef]
- Tu, H.; Li, D.; Yi, Y.; Liu, R.; Wu, Y.; Dong, X.; Shi, X.; Deng, H. Incorporation of rectorite into porous polycaprolactone/TiO2 nanofibrous mats for enhancing photocatalysis properties towards organic dye pollution. Compos. Commun. 2019, 15, 58–63. [Google Scholar] [CrossRef]
- Korina, E.; Stoilova, O.; Manolova, N.; Rashkov, I. Multifunctional hybrid materials from poly(3-hydroxybutyrate), TiO2 nanoparticles, and chitosan oligomers by combining electrospinning/electrospraying and impregnation. Macromol. Biosci. 2013, 13, 707–716. [Google Scholar] [CrossRef]
- Bergamonti, L.; Bergonzi, C.; Graiff, C.; Lottici, P.P.; Bettini, R.; Elviri, L. 3D printed chitosan scaffolds: A new TiO2 support for the photocatalytic degradation of amoxicillin in water. Water Res. 2019, 163, 114841. [Google Scholar] [CrossRef] [PubMed]
- Ikhlef-Taguelmimt, T.; Hamiche, A.; Yahiaoui, I.; Bendellali, T.; Lebik-Elhadi, H.; Ait-Amar, H.; Aissani-Benissad, F. Tetracycline hydrochloride degradation by heterogeneous photocatalysis using TiO2(P25) immobilized in biopolymer (chitosan) under UV irradiation. Water Sci. Technol. 2020, 82, 1570–1578. [Google Scholar] [CrossRef]
- Nouri, L.; Hemidouche, S.; Boudjemaa, A.; Kaouah, F.; Sadaoui, Z.; Bachari, K. Elaboration and characterization of photobiocomposite beads, based on titanium (IV) oxide and sodium alginate biopolymer, for basic blue 41 adsorption/photocatalytic degradation. Int. J. Biol. Macromol. 2020, 151, 66–84. [Google Scholar] [CrossRef]
- Bobirică, C.; Bobirică, L.; Râpă, M.; Matei, E.; Predescu, A.M.; Orbeci, C. Photocatalytic degradation of ampicillin using PLA/TiO2 hybrid nanofibers coated on different types of fiberglass. Water 2020, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Shao, Q.; Zhao, J.R.B.; Pan, D.; Dong, M.; Jia, C.; Ding, T.; Wu, T.; Guo, Z. Microwave solvothermal carboxymethyl chitosan templated synthesis of TiO 2 /ZrO 2 composites toward enhanced photocatalytic degradation of Rhodamine B. J. Colloid Interface Sci. 2019, 541, 18–29. [Google Scholar] [CrossRef]
- Lei, P.; Wang, F.; Gao, X.; Ding, Y.; Zhang, S.; Zhao, J.; Liu, S.; Yang, M. Immobilization of TiO2 nanoparticles in polymeric substrates by chemical bonding for multi-cycle photodegradation of organic pollutants. J. Hazard. Mater. 2012, 227–228, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Midya, L.; Sarkar, A.N.; Das, R.; Maity, A.; Pal, S. Crosslinked chitosan embedded TiO2 NPs and carbon dots-based nanocomposite: An excellent photocatalyst under sunlight irradiation. Int. J. Biol. Macromol. 2020, 164, 3676–3686. [Google Scholar] [CrossRef] [PubMed]
- Erim, B.; Ciğeroğlu, Z.; Bayramoğlu, M. Green synthesis of TiO2/GO/chitosan by using leaf extract of Olea europaea as a highly efficient photocatalyst for the degradation of cefixime trihydrate under UV-A radiation exposure: An optimization study with D-optimal design. J. Mol. Struct. 2021, 1234. [Google Scholar] [CrossRef]
- ZabihiSahebi, A.; Koushkbaghi, S.; Pishnamazi, M.; Askari, A.; Khosravi, R.; Irani, M. Synthesis of cellulose acetate/chitosan/SWCNT/Fe3O4/TiO2 composite nanofibers for the removal of Cr(VI), As(V), Methylene blue and Congo red from aqueous solutions. Int. J. Biol. Macromol. 2019, 140, 1296–1304. [Google Scholar] [CrossRef]
- Bahrudin, N.N.; Nawi, M.A.; Zainal, Z. Insight into the synergistic photocatalytic-adsorptive removal of methyl orange dye using TiO2/chitosan based photocatalyst. Int. J. Biol. Macromol. 2020, 165, 2462–2474. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.; Gao, F.; Mailhot, G.; Pan, G. Algae decorated TiO2/Ag hybrid nanofiber membrane with enhanced photocatalytic activity for Cr(VI) removal under visible light. Chem. Eng. J. 2017, 314, 622–630. [Google Scholar] [CrossRef] [Green Version]
- Morlando, A.; Sencadas, V.; Cardillo, D.; Konstantinov, K. Suppression of the photocatalytic activity of TiO2 nanoparticles encapsulated by chitosan through a spray-drying method with potential for use in sunblocking applications. Powder Technol. 2018, 329, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhu, X.; Wang, L.; Wu, F.; Liu, S.; Chang, C.; Luo, X. A Simple Strategy to Design 3-Layered Au-TiO2 Dual Nanoparticles Immobilized Cellulose Membranes with Enhanced Photocatalytic Activity. Carbohydr. Polym. 2020, 231. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Salleh, W.W.N.; Jaafar, J.; Ismail, A.F.; Mutalib, M.A.; Sani, N.A.A.; Asri, S.E.A.M.; Ong, C.S. Physicochemical characteristic of regenerated cellulose/N-doped TiO2 nanocomposite membrane fabricated from recycled newspaper with photocatalytic activity under UV and visible light irradiation. Chem. Eng. J. 2016, 284, 202–215. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Yousefi Kebria, D.; Qaderi, F. Effect of biosurfactant as a novel draw solution on photocatalytic treatment and desalination of produced water by different forward osmosis membranes. Water Sci. Technol. Water Supply 2020, 20, 240–250. [Google Scholar] [CrossRef]
- Lin, Z.; Lu, Y.; Huang, J. A hierarchical Ag2O-nanoparticle/TiO2-nanotube composite derived from natural cellulose substance with enhanced photocatalytic performance. Cellulose 2019, 26, 6683–6700. [Google Scholar] [CrossRef]
- Ullah, S.; Acuña, J.J.S.; Pasa, A.A.; Bilmes, S.A.; Vela, M.E.; Benitez, G.; Rodrigues-Filho, U.P. Photoactive layer-by-layer films of cellulose phosphate and titanium dioxide containing phosphotungstic acid. Appl. Surf. Sci. 2013, 277, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Wittmar, A.S.M.; Fu, Q.; Ulbricht, M. Photocatalytic and magnetic porous cellulose macrospheres for water purification. Cellulose 2019, 26, 4563–4578. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Zhang, B.; Liu, R. Adsorption and photocatalytic activity of Cu-doped cellulose nanofibers/nano-titanium dioxide for different types of dyes. Water Sci. Technol. 2020, 82, 1665–1675. [Google Scholar] [CrossRef]
- Lan, Y.; Yu, M.; He, D.; Wang, Y.; Meng, Q.B.; Huang, H.; Zhang, Y.; Ma, T.; Song, X.M. Pickering emulsion-embedded hierarchical solid-liquid hydrogel spheres for static and flow photocatalysis. J. Colloid Interface Sci. 2021, 589, 587–596. [Google Scholar] [CrossRef]
- Nawaz, M.; Khan, A.A.; Hussain, A.; Jang, J.; Jung, H.Y.; Lee, D.S. Reduced graphene oxide−TiO2/sodium alginate 3-dimensional structure aerogel for enhanced photocatalytic degradation of ibuprofen and sulfamethoxazole. Chemosphere 2020, 261, 127702. [Google Scholar] [CrossRef] [PubMed]
- Dalponte, I.; de Sousa, B.C.; Mathias, A.L.; Jorge, R.M.M. Formulation and optimization of a novel TiO2/calcium alginate floating photocatalyst. Int. J. Biol. Macromol. 2019, 137, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Tian, Q.; Sun, Q.; Wei, W.; Zhuang, J.; Liu, M.; Cao, Z.; Xie, W.; Fan, M. TiO2-alginate composite aerogels as novel oil/water separation and wastewater remediation filters. Compos. Part B Eng. 2019, 160, 480–487. [Google Scholar] [CrossRef]
- Abdel Rehim, M.H.; El-Samahy, M.A.; Badawy, A.A.; Mohram, M.E. Photocatalytic activity and antimicrobial properties of paper sheets modified with TiO2/Sodium alginate nanocomposites. Carbohydr. Polym. 2016, 148, 194–199. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Favvas, E.P.; Romanos, G.E.; Athanasekou, C.P.; Beltsios, K.G.; Tzialla, O.I.; Falaras, P. Alginate fibers as photocatalyst immobilizing agents applied in hybrid photocatalytic/ultrafiltration water treatment processes. Water Res. 2012, 46, 1858–1872. [Google Scholar] [CrossRef]
- Lin, D.; Huang, Y.; Liu, Y.; Luo, T.; Xing, B.; Yang, Y.; Yang, Z.; Wu, Z.; Chen, H.; Zhang, Q.; et al. Physico-mechanical and structural characteristics of starch/polyvinyl alcohol/nano-titania photocatalytic antimicrobial composite films. Lwt 2018, 96, 704–712. [Google Scholar] [CrossRef]
- Muniandy, S.S.; Mohd Kaus, N.H.; Jiang, Z.T.; Altarawneh, M.; Lee, H.L. Green synthesis of mesoporous anatase TiO2 nanoparticles and their photocatalytic activities. RSC Adv. 2017, 7, 48083–48094. [Google Scholar] [CrossRef] [Green Version]
- Warkhade, S.K.; Gaikwad, G.S.; Zodape, S.P.; Pratap, U.; Maldhure, A.V.; Wankhade, A.V.L. Temperature synthesis of pure anatase carbon doped titanium dioxide: A. efficient visible light active photocatalyst Low temperature synthesis of pure anatase carbon doped titanium dioxide: An efficient visible light active photocatalyst. Mater. Sci. Semicond. Process. 2017, 63, 18–24. [Google Scholar] [CrossRef]
- Toor, A.P.; Yadav, N.; Wanchoo, R.K. Enhancement in photocatalytic activity of nano-TiO2 photocatalyst by carbon doping. Mater. Sci. Forum 2013, 757, 271–284. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, Z.; Yan, J.; Qiang, L.; Ru, X.; Liu, F.; Ding, D.; Li, J. Preparation and characterization of TiO2 nanofibers via using polylactic acid as template. J. Appl. Polym. Sci. 2013, 128, 1095–1100. [Google Scholar] [CrossRef]
- Eleftheriadou, N.M.; Ofrydopoulou, A.; Papageorgiou, M.; Lambropoulou, D. Development of novel polymer supported nanocomposite GO/TiO2 films, based on poly(L-lactic acid) for photocatalytic applications. Appl. Sci. 2020, 10, 2368. [Google Scholar] [CrossRef] [Green Version]
- Evgenidou, E.; Ofrydopoulou, A.; Malesic-Eleftheriadou, N.; Nannou, C.; Ainali, N.M.; Christodoulou, E.; Bikiaris, D.N.; Kyzas, G.Z.; Lambropoulou, D.A. New insights into transformation pathways of a mixture of cytostatic drugs using Polyester-TiO2 films: Identification of intermediates and toxicity assessment. Sci. Total Environ. 2020, 741. [Google Scholar] [CrossRef]
- Karagoz, S.; Kiremitler, N.B.; Sakir, M.; Salem, S.; Onses, M.S.; Sahmetlioglu, E.; Ceylan, A.; Yilmaz, E. Synthesis of Ag and TiO2 modified polycaprolactone electrospun nanofibers (PCL/TiO2-Ag NFs) as a multifunctional material for SERS, photocatalysis and antibacterial applications. Ecotoxicol. Environ. Saf. 2020, 188. [Google Scholar] [CrossRef] [PubMed]
- Sivlim, T.; Akkan, Ş.; AltIn, I.; Koç, M.; Sökmen, M. TiO2 immobilized biodegradable polymer for photocatalytic removal of chlorophenol. Water. Air. Soil Pollut. 2012, 223, 3955–3964. [Google Scholar] [CrossRef]
- Marković, D.; Milovanović, S.; Radovanović, Ž.; Zizovic, I.; Šaponjić, Z.; Radetić, M. Floating Photocatalyst Based on Poly(ε-caprolactone) Foam and TiO2 Nanoparticles for Removal of Textile Dyes. Fibers Polym. 2018, 19, 1219–1227. [Google Scholar] [CrossRef]
- Pelaseyed, S.S.; Hosseini, H.R.M.; Nokhbedehghan, Z.; Samadikuchaksaraei, A. PLGA/TiO2 nanocomposite scaffolds for biomedical applications: Fabrication, photocatalytic, and antibacterial properties. BioImpacts 2020, 11, 45–52. [Google Scholar] [CrossRef]











| Biodegradable Polymeric Matrix | Photocatalysis Parameters | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Polymer Substrate | TiO2 Precursor | Dopant | Immobilization Technique | Morphology of the Photocatalyst | Type of (Target) Pollutant | Light source | Degradation Efficiency (Time Required) | |
| 1 | Calcium alginate | TiO2 (P25, 20% rutile and 80% anatase, ≥99%) | GO | One-step emulsion gelation method | Hierarchical solid-liquid gel spheres | 2-naphthol Rhodamine B | A xenon lamp with power 500 W, and light intensity 100 mW/cm2 | 50% (150 min) 58% (150 min) | [33] |
| 2 | SA | TiO2 nanopowders, anatase phase | GO | Simple hydrothermal treatment method | 3D sodium alginate/graphene oxide/TiO2 aerogel | Ibuprofen (IBUP) Sulfamethoxazole (SMX) | UV-A light through a photo-reactor with light 13.5 ± 1 W/m2 | ~78% (90 min) ~90% (45 min) | [34] |
| 3 | CaAlg | Titanium (IV) oxide, anatase powder (99.8% trace metals basis) | - | Extrusion method | Biocomposite beads | Basic blue 41 | Cylindrical jacketed batch reactor with simulated sunlight irradiation 90 W/cm2 | ~96% (240 min) | [16] |
| 4 | CaAlg | TiO2 nanopowder P-25 | - | -Dripping method -Ionic gelation | Buoyant TiO2/CaAlg photocatalyst | Tartrazine | UV 125 W), λ = 254 nm | >89% (180 min) | [35] |
| 5 | SA | P25 (nanoscale TiO2 powder) | - | - Homogeneous dispersion solution - Cross-linking | TiO2-alginate composite aerogels as | Methyl orange | Simulated sunlight irradiation, 300 W xenon lamp | >96.7% (150 min) | [36] |
| 6 | SA | Titanium tert-butoxide | - | Precipitation method | Paper sheets modified with TiO2/Sodium alginate nanocomposites | COD | UV light lamp with λ = 246 nm | 42–18% (120 min) | [37] |
| 7 | CaAlg | AEROXIDE® TiO2 P25 | - | Dry/wet spinning process | CaAlg/TiO2 fibers | Methyl orange | Four UV lamps, PL-S/PL-L, 9 W), with λ = 315–380 nm | ~98–90% (340–272 min) | [38] |
| Biodegradable Polymeric Matrix. | Photocatalysis Parameters | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Polymer Substrate | TiO2 Precursor | Dopant | Immobilization Technique | Morphology of the Photocatalyst | Type of (Target) Pollutant | Light Source | Degradation Efficiency (Time Required) | |
| 1 | CA PCL PLA | Aeroxide® P25 TiO2 nanoparticles | - | Solution casting method | Composite films | Methylene blue (MB) | UV-A light system fitted with four 40 W lamps | 72% (180 min) | [3] |
| 2 | CS + PLA | Titanium dioxide (Degussa P90) | - | - Sol-gel method, - step wise spin-coating method | Hybrid Multilayer Coated films | Methyl orange | UV light radiation under a UV instrument | ~80% (600 min) | [6] |
| 3 | PLA | TiO2 nanoparticles (anatase) | - | Electrospinning | Membranes of hybrid nanofibers | Ampicillin | UV light through a lamp 120 W | 54–34% (30 min) | [17] |
| 4 | PLA | Tetrabutyl titanate (TBT, 98% pure) | - | Electrospinning | TiO2/PLA composite nanofibers | Methylene orange | UV lamp, λ = 375 nm | ~40% (240 min) | [43] |
| 5 | PLLA | Titanium dioxide P25 (~80% anatase and 20% rutile) | - | Phase inversion method | GO/TiO2 PLLA-supported nanocomposite films | Sulfamethoxazole Sulfadiazine Levofloxacin Norfloxacin Moxifloxacin Isoniazid Metronidazole Lincomycin Trimethoprim | Simulated solar irradiation through Suntest Atlas CPS+ solar with a xenon lamp (1.5 W and 750 W/m2) | >90% for most of antibiotics (120–360 min) | [44] |
| Biodegradable Polymeric Matrix | Photocatalysis Parameters | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Polymer Substrate | TiO2 Precursor | Dopant | Immobilization Technique | Morphology of the photocatalyst | Type of (Target) Pollutant | Light Source | Degradation Efficiency (Time Required) | |
| 1 | PCL | AEROXIDE® TiO2 P25 nanopowder | Ag | One-step electrospinning | Ag and TiO2 modified PCL electrospun nanofiber mats | Methylene blue Ibuprofen E. Coli Staphylococcus aureus | UV lamp with wavelength 380 nm and intensity 400 W | 93–90% (160 min) 50% (50 min) - | [46] |
| 2 | PCL | TiO2 nanoparticles (P25) | REC | Electrospinning | PCL/TiO2/REC porous mats | Rhodamine B | Exposure to a UV lamp with λ = 254 nm and intensity 25 W | 98% (240 min) | [12] |
| 3 | PCL | TiO2 powder | - | Electrospinning & plasma treatment | PCL/TiO2 nanofiber mats | Reactive Black 5 | UV lamps with λ = 254 nm | ~50% (120 min) | [11] |
| 4 | PCL | n-TiO2 powder | - | Solvent-cast process | n-TiO2 immobilized PCL films | Methylene blue, C. albicans | UVA lamp (365 nm) used or a visible light emitting sodium lamp | 94% (150 min) 54% (60 min) | [5] |
| 5 | PCL | n-TiO2 powder | - | Solvent-cast process | n-TiO2 immobilized PCL films | 4-chlorophenol | UV lamp (Kolorlu× H400/40 400 W 0303 Hungary, λ = 365 nm | 45–20% (150 min) | [47] |
| 6 | PCL | TiO2 NPs (Degussa P-25) | - | Supercritical Foaming & immobilization of TiO2 onto PCL foams | PCL foams with immobilized TiO2 | Acid Orange 7 Basic Yellow 28 | Exposure to an ULTRA-VITALUX lamp 300 W, simulated sunlight irradiation | 100% (24 h) 100% (24 h) | [48] |
| Biodegradable Polymeric Matrix | Photocatalysis Parameters | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Polymer Substrate | TiO2 Precursor | Dopant | Immobilization technique | Morphology of the Photocatalyst | Type of (Target) Pollutant | Light Source | Degradation Efficiency (Time Required) | |
| 1 | PLA + PBAT + PBS | Titanium isopropoxide (97 wt%) | - | - Sol-gel method for theTiO2 nanoparticles - blown film technique | Composite films | Toluene | Photocatalytic oxidation reactor with UV-C lamp 6 W and λ = 254 nm | 52% (270 min) | [7] |
| 2 | PLGA | TiO2 nanopowder | - | Air-liquid foaming technique | Porous 3D-PCL scaffolds | Methylene blue E. Coli | UV lamp light with wavelength 365 nm | 90% (180 min) ~99% (24 h) | [49] |
| 3 | PHB & CS oligomers | Titanium (IV) oxide (nano- TiO2) (99.7% anatase nanopowder) | - | Electrospinning/Electrospraying & Impregnation techniques | Hybrid fibrous materials | Methylene Blue Escherichia Coli | UV light (UVASPOT 400/T, Dr. Honle AG; UV lamp UV 400 F/2; 400 W) | >92% (180 min) 100% (30–60 min) | [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ainali, N.M.; Kalaronis, D.; Evgenidou, E.; Bikiaris, D.N.; Lambropoulou, D.A. Insights into Biodegradable Polymer-Supported Titanium Dioxide Photocatalysts for Environmental Remediation. Macromol 2021, 1, 201-233. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1030015
Ainali NM, Kalaronis D, Evgenidou E, Bikiaris DN, Lambropoulou DA. Insights into Biodegradable Polymer-Supported Titanium Dioxide Photocatalysts for Environmental Remediation. Macromol. 2021; 1(3):201-233. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1030015
Chicago/Turabian StyleAinali, Nina Maria, Dimitrios Kalaronis, Eleni Evgenidou, Dimitrios N. Bikiaris, and Dimitra A. Lambropoulou. 2021. "Insights into Biodegradable Polymer-Supported Titanium Dioxide Photocatalysts for Environmental Remediation" Macromol 1, no. 3: 201-233. https://0-doi-org.brum.beds.ac.uk/10.3390/macromol1030015