Resolvin D1, a Metabolite of Omega-3 Polyunsaturated Fatty Acid, Decreases Post-Myocardial Infarct Depression
Abstract
:1. Introduction
2. Results
2.1. Infarct Size

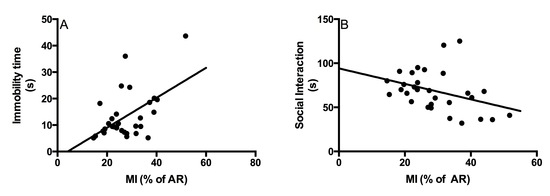
2.2. Behavioral Tests
2.2.1. Social Interaction Test


2.2.2. Forced Swim Test


3. Methods
3.1. Experimental Design
3.2. Surgical Procedure
3.2.1. Behavioral Measures
3.2.2. Social Interaction Test
3.2.3. Forced Swim Test
3.2.4. Measurement of Infarct Size
3.3. Statistical Analysis
4. Discussion
5. Limitations
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meneses, R.; Almeida, M.C.; Abecasis, J.; Arroja, I.; Carvalho, A.; Aleixo, A. Depression in patients with myocardial infarction. Rev. Port. Cardiol. 2007, 26, 1143–1165. [Google Scholar]
- Frasure-Smith, N.; Lespérance, F.; Talajic, M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993, 270, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Bah, T.M.; Benderdour, M.; Kaloustian, S.; Karam, R.; Rousseau, G.; Godbout, R. Escitalopram reduces circulating pro-inflammatory cytokines and improves depressive behavior without affecting sleep in a rat model of post-cardiac infarct depression. Behav. Brain Res. 2011, 225, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Wann, B.P.; Bah, T.M.; Kaloustian, S.; Boucher, M.; Dufort, A.M.; le Marec, N.; Godbout, R.; Rousseau, G. Behavioural signs of depression and apoptosis in the limbic system following myocardial infarction: Effects of sertraline. J. Psychopharmacol. 2009, 23, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Arseneault-Bréard, J.; Rondeau, I.; Gilbert, K.; Girard, S.A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012, 107, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, K.; Arseneault-Breard, J.; Flores Monaco, F.; Beaudoin, A.; Bah, T.M.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Attenuation of post-myocardial infarction depression in rats by n-3 fatty acids or probiotics starting after the onset of reperfusion. Br. J. Nutr. 2013, 109, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bah, T.M.; Kaloustian, S.; Rousseau, G.; Godbout, R. Pretreatment with pentoxifylline has antidepressant-like effects in a rat model of acute myocardial infarction. Behav. Pharmacol. 2011, 22, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, G.; Bah, T.M.; Godbout, R. Post-myocardial infarction depression. In Novel Strategies in Ischemic Heart Disease; Lakshmanadoss, U., Ed.; InTech: Rijeka, Croatia, 2012; pp. 333–362. [Google Scholar]
- Francis, J.; Chu, Y.; Johnson, A.K.; Weiss, R.M.; Felder, R.B. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am. J. Physiol. 2004, 286, H2264–H2271. [Google Scholar]
- Kaloustian, S.; Bah, T.M.; Rondeau, I.; Mathieu, S.; Lada-Moldovan, L.; Ryvlin, P.; Godbout, R.; Rousseau, G. Tumor necrosis factor-alpha participates in apoptosis in the limbic system after myocardial infarction. Apoptosis 2009, 14, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Van den Biggelaar, A.H.; Gussekloo, J.; de Craen, A.J.; Frolich, M.; Stek, M.L.; van der Mast, R.C.; Westendorp, R.G. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp. Gerontol. 2007, 42, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Novel eicosanoid and docosanoid mediators: Resolvins, docosatrienes, and neuroprotectins. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Spite, M.; Serhan, C.N. Novel lipid mediators promote resolution of acute inflammation: Impact of aspirin and statins. Cir. Res. 2010, 107, 1170–1184. [Google Scholar] [CrossRef]
- Serhan, C.N.; Arita, M.; Hong, S.; Gotlinger, K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids 2004, 39, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Gotlinger, K.; Hong, S.; Arita, M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: An overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004, 73, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Ohira, T.; Arita, M.; Omori, K.; Recchiuti, A.; van Dyke, T.E.; Serhan, C.N. Resolvin e1 receptor activation signals phosphorylation and phagocytosis. J. Biol. Chem. 2010, 285, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, M.K.; Lee, E.J.; Lee, C.H. Resolvin D1 inhibits TGF-beta1-induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32. Int. J. Biochem. Cell Biol. 2013, 45, 2801–2807. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N. Endogenous pro-resolving and anti-inflammatory lipid mediators: A new pharmacologic genus. Br. J. Pharmacol. 2008, 153, S200–S215. [Google Scholar] [CrossRef] [PubMed]
- Tran Quang, T.; Gosselin, A.A.; Bourque-Riel, V.; Gilbert, K.; Charron, T.; Rousseau, G. Effect of resolvin d1 on experimental myocardial infarction. Exp. Clin. Cardiol. 2014, in press. [Google Scholar]
- Wann, B.P.; Bah, T.M.; Boucher, M.; Courtemanche, J.; le Marec, N.; Rousseau, G.; Godbout, R. Vulnerability for apoptosis in the limbic system after myocardial infarction in rats: A possible model for human postinfarct major depression. J. Psychiatry Neurosci. 2007, 32, 11–16. [Google Scholar] [PubMed]
- Simpson, P.J.; Fantone, J.C.; Mickelson, J.K.; Gallagher, K.P.; Lucchesi, B.R. Identification of a time window for therapy to reduce experimental canine myocardial injury: Suppression of neutrophil activation during 72 h of reperfusion. Circ. Res. 1988, 63, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Das, D.K. Role of cytokines in myocardial ischemia and reperfusion. Mediat. Inflamm. 1997, 6, 175–183. [Google Scholar] [CrossRef]
- Ren, G.; Dewald, O.; Frangogiannis, N.G. Inflammatory mechanisms in myocardial infarction. Curr. Drug Targets Inflamm. Allergy 2003, 2, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Nah, D.Y.; Rhee, M.Y. The inflammatory response and cardiac repair after myocardial infarction. Korean Circ. J. 2009, 39, 393–398. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M.; Rousseau, G.; Basmadjian, A.; St-Jean, G.; Tran, D.C.; Latour, J.G. Spacial and temporal profiles of neutrophil accumulation in the reperfused ischemic myocardium. Am. J. Cardiovasc. Pathol. 1990, 3, 143–154. [Google Scholar]
- Mullane, K.M.; Kraemer, R.; Smith, B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J. Pharmacol. Methods 1985, 14, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Kaloustian, S.; Wann, B.P.; Bah, T.M.; Girard, S.A.; Apostolakis, A.; Ishak, S.; Mathieu, S.; Ryvlin, P.; Godbout, R.; Rousseau, G. Apoptosis time course in the limbic system after myocardial infarction in the rat. Brain Res. 2008, 1216, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.A.; Bah, T.M.; Kaloustian, S.; Lada-Moldovan, L.; Rondeau, I.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Lactobacillus helveticus and Bifidobacterium longum taken in combination reduce the apoptosis propensity in the limbic system after myocardial infarction in a rat model. Br. J. Nutr. 2009, 102, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Baxter, G.F.; Burley, D.S. Reperfusion and calculated risks: Pharmacological postconditioning of human myocardium. Br. J. Pharmacol. 2008, 153, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, A.K.; Sack, M.N.; Mjos, O.D.; Yellon, D.M. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ. Res. 2001, 89, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Boucher, M.; Pesant, S.; Falcao, S.; de Montigny, C.; Schampaert, E.; Cardinal, R.; Rousseau, G. Post-ischemic cardioprotection by A2A adenosine receptors: Dependent of phosphatidylinositol 3-kinase pathway. J. Cardiovasc. Pharmacol. 2004, 43, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Bopassa, J.C.; Ferrera, R.; Gateau-Roesch, O.; Couture-Lepetit, E.; Ovize, M. Pi 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. Cardiovasc. Res. 2006, 69, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Bah, T.M.; Wann, B.P.; Chebli, M.; le Marec, N.; Rousseau, G.; Godbout, R. Insomnia and increased rem sleep pressure in a rat model of post myocardial infarction depression. Can. Sleep Soc. 2007, in press. [Google Scholar]
- Bah, T.M.; Laplante, F.; Wann, B.P.; Sullivan, R.; Rousseau, G.; Godbout, R. Paradoxical sleep insomnia and decreased cholinergic neurons after myocardial infarction in rats. Sleep 2010, 33, 1703–1710. [Google Scholar] [PubMed]
- Keyes, K.T.; Ye, Y.; Lin, Y.; Zhang, C.; Perez-Polo, J.R.; Gjorstrup, P.; Birnbaum, Y. Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. 2010, 299, H153–H164. [Google Scholar]
- Al-Zakwani, I.; Zubaid, M.; Al-Riyami, A.; Alanbaei, M.; Sulaiman, K.; Almahmeed, W.; Al-Motarreb, A.; Al Suwaidi, J. Primary coronary intervention versus thrombolytic therapy in myocardial infarction patients in the middle east. Int. J. Clin. Pharm. 2012, 34, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Rymuza, H.; Kowalik, I.; Drzewiecki, A.; Krzyzanowski, W.; Olszewski, M.; Dabrowski, R.; Jedrzejczyk, B.; Wozniak, J.; Sosnowski, C.; Szwed, H. Successful primary coronary angioplasty improves early and long-term outcomes in st segment elevation acute coronary syndromes in patients above 80 years of age. Kardiol. Pol. 2011, 69, 346–354. [Google Scholar] [PubMed]
- Westerhout, C.M.; Bonnefoy, E.; Welsh, R.C.; Steg, P.G.; Boutitie, F.; Armstrong, P.W. The influence of time from symptom onset and reperfusion strategy on 1-year survival in st-elevation myocardial infarction: A pooled analysis of an early fibrinolytic strategy versus primary percutaneous coronary intervention from captim and west. Am. Heart J. 2011, 161, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.L.; Han, Y.L.; Yang, X.C.; Mao, J.M.; Fang, W.Y.; Wang, L.; Shen, W.F.; Li, Z.Q.; Jia, G.L.; Lu, S.Z.; et al. Thorombolytic therapy with rescue percutaneous coronary intervention versus primary percutaneous coronary intervention in patients with acute myocardial infarction: A multicenter randomized clinical trial. Chin. Med. J. 2010, 123, 1365–1372. [Google Scholar] [PubMed]
- Borgia, F.; Goodman, S.G.; Halvorsen, S.; Cantor, W.J.; Piscione, F.; le May, M.R.; Fernandez-Aviles, F.; Sanchez, P.L.; Dimopoulos, K.; Scheller, B.; et al. Early routine percutaneous coronary intervention after fibrinolysis vs. Standard therapy in ST-segment elevation myocardial infarction: A meta-analysis. Eur. Heart J. 2010, 31, 2156–2169. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilbert, K.; Bernier, J.; Godbout, R.; Rousseau, G. Resolvin D1, a Metabolite of Omega-3 Polyunsaturated Fatty Acid, Decreases Post-Myocardial Infarct Depression. Mar. Drugs 2014, 12, 5396-5407. https://0-doi-org.brum.beds.ac.uk/10.3390/md12115396
Gilbert K, Bernier J, Godbout R, Rousseau G. Resolvin D1, a Metabolite of Omega-3 Polyunsaturated Fatty Acid, Decreases Post-Myocardial Infarct Depression. Marine Drugs. 2014; 12(11):5396-5407. https://0-doi-org.brum.beds.ac.uk/10.3390/md12115396
Chicago/Turabian StyleGilbert, Kim, Judith Bernier, Roger Godbout, and Guy Rousseau. 2014. "Resolvin D1, a Metabolite of Omega-3 Polyunsaturated Fatty Acid, Decreases Post-Myocardial Infarct Depression" Marine Drugs 12, no. 11: 5396-5407. https://0-doi-org.brum.beds.ac.uk/10.3390/md12115396