Genista tridentata L.: A Rich Source of Flavonoids with Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Genista tridentata: Traditional Applications and Biological Activities
3. Structural Pattern of the Flavonoids Isolated from Genista tridentata
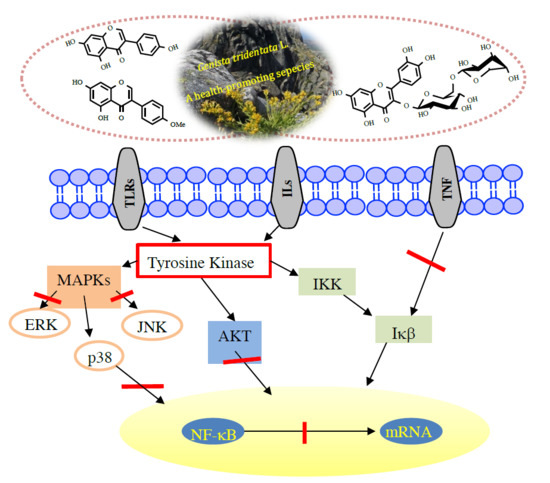
4. Flavonoids with Anti-Inflammatory Activity
4.1. Biochanin A and Prunetin
4.2. Daidzein
4.3. Genistein
4.4. Rutin
4.5. Taxifolin
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysm |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| Ag | antigen |
| AKT | serine/threonine kinase |
| Bax | Bcl-2 associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| Bcl-3 | B-cell lymphoma-3 |
| BMMCs | bone marrow derived mast cells |
| caspase-3 | cysteine aspartate specific protease-3 |
| CCL2 | chemokine ligand 2 |
| CLP | cecal ligation and puncture |
| CRP | C-reactive protein |
| CXC | α-chemokines |
| CYP450 | Cytochrome P450 |
| DPPH● | 2,2-diphenyl-1-picrylhydrazyl radical |
| DSS | dextran sulfate sodium |
| E-selection | endothelial cells |
| ERK | extracellular signal-regulated protein kinase |
| G. | Genista |
| GalN | D-galactosamine |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GPx | glutathione peroxidase |
| HMEC-1 | human dermal microvascular endothelial cell-1 |
| HMGB1 | high mobility group box 1 |
| HO-1 | heme oxygenase-1 |
| HUVEC | human umbilical vein endothelial |
| ICAM-1 | intercellular adhesion molecule-1 |
| IFNγ | interferon gamma |
| IgE | immunoglobulin E |
| IKK | IκB kinase |
| IL-10 | interleukin-10 |
| IL-12 | interleukin-12 |
| IL-18 | interleukin-18 |
| IL-1α | interleukin-1α |
| IL-1β | interleukin-1β |
| IL-2 | interleukin-2 |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| iNOS | inducible nitric oxide synthase |
| JNK | c-jun N-terminal kinase |
| LIX | lipopolysaccharide-induced CXC chemokine |
| LPS | lipopolysaccharides |
| LTC4 | cysteinyl leukotriene 4 |
| MAPK | mitogen-activated protein kinases |
| MCP-1 | monocyte chemoattractant protein-1 |
| MDA | malondialdehyde |
| MIP-3α | macrophage inflammatory protein 3 α |
| MMP | matrix metalloproteinases |
| MPO | myeloperoxidase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MUC5AC | mucin 5AC glycoprotein |
| MyD88 | myeloid differentiation primary response 88 |
| NASH | nonalcoholic steatohepatitis |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NFATc1 | nuclear factor-activated T cells c1 |
| NLRP3 | NRL pyrin domain containing 3 |
| Nos2 | nitric oxide synthase 2 |
| Nrf2 | nuclear factor erythroid 2 |
| NRL | nucleotide-binding, leucine-rich repeat containing proteins |
| PGE2 | prostaglandin E2 |
| PMA | phorbol 12-myristate 13-acetate |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| Ptgs2 | prostaglandin-endoperoxide synthase 2 |
| RAGE | receptor for advanced glycation end-products |
| RANKL | receptor activator of nuclear factor-κB ligand |
| SIRT1 | sirtuin 1 |
| SOD | superoxide dismutase |
| sTNFR1 | soluble tumor necrosis factor receptor-1 |
| TBARS | thiobarbituric acid reactive substances |
| TEER | transepithelial electrical resistance |
| TGF-β1 | transforming growth factor β1 |
| TLR 4 | toll-like receptors 4 |
| TNF-α | tumor necrosis factor alpha |
| TRAP | tartrate-resistant acid phospha- tase |
| TXNIP | thioredoxin-interacting protein |
| VCAM-1 | vascular cytoadhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
| VEGFA | vascular endothelial growth factor A |
| VSMC | vascular smooth muscle cells |
References
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Tech. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef]
- Neves, J.M.; Matos, C.M.; Moutinho, C.G.; Gomes, L.R. Usos Populares de Plantas Medicinais da Flora Transmontana; Edições Universidade Fernando Pessoa: Porto, Portugal, 2008; pp. 226–235. [Google Scholar]
- Novais, M.H.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on pharmaceutical ethnobotany in Arrabida Natural Park (Portugal). J. Ethnopharm. 2004, 93, 183–195. [Google Scholar] [CrossRef]
- Ferrándiz, M.L.; Alcaraz, M.J. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions 1991, 32, 283–288. [Google Scholar] [CrossRef]
- Simões, M.A.M.; Pinto, D.C.G.A.; Neves, B.M.R.; Silva, A.M.S. Flavonoid profile of the Genista tridentata L., a species used traditionally to treat inflammatory processes. Molecules 2020, 25, 812. [Google Scholar] [CrossRef] [Green Version]
- Hämäläinen, M.; Nieminen, R.; Vuorela, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Q.; Jin, Z.Y.; Li, G.H. Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage through inhibition of microglia activation and proinflammatory factors generation. Neurosci. Lett. 2007, 417, 112–117. [Google Scholar] [CrossRef]
- Tan, J.W.; Kim, M.K. Neuroprotective effects of biochanin A against b-amyloid-induced neurotoxicity in PC12 cells via a mitochondrial-dependent apoptosis pathway. Molecules 2016, 21, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, G.; Pereira, A.L. Winged stems in Pterospartum tridentatum: Morphoanatomical study. Acta Bot. Gall. 2004, 151, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Etgnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283. [Google Scholar] [CrossRef] [PubMed]
- The Plant List Database. Available online: http://www.theplantlist.org/ (accessed on 26 May 2020).
- Carvalho, A.M. Plantas y sabiduría popular del Parque Natural de Montesinho: Un estudio etnobotánico en Portugal. In Biblioteca de Ciencias No 35; Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2010; p. 496. [Google Scholar]
- Flora-On. Available online: https://flora-on.pt/index.php#/0BJwn (accessed on 26 May 2020).
- Camejo-Rodrigues, J.; Ascensão, L.; Bonet, M.À.; Vallès, J. An ethnobotanical study of medicinal and aromatic plants in the natural park of “Serra de São Mamede”)Portugal). J. Ethnopharmacol. 2003, 89, 199–209. [Google Scholar] [CrossRef]
- Rivera, D.; Verde, J.; Fajardo, J.; Obón, C.; Consuegra, V.; García-Botía, J.; Ríos, S.; Alcaraz, F.; Valdés, A.; del Moral, A.; et al. Ethnopharmacology in the upper Guadiana river área (Castile-La Mancha, Spain). J. Ethnopharmacol. 2019, 241, 111968. [Google Scholar] [CrossRef]
- Vitor, R.F.; Mota-Filipe, H.; Teixeira, G.; Borges, C.; Rodrigues, A.I.; Teixeira, A.; Paulo, A. Flavonoids of na extract of Pterospartum tridentatum showing endothelial protection against oxidative injury. J. Ethnopharmacol. 2004, 93, 363–370. [Google Scholar] [CrossRef]
- Grosso, A.C.; Costa, M.M.; Ganço, L.; Pereira, A.L.; Teixeira, G.; Lavado, J.M.G.; Figueireido, A.C.; Pedro, L.G. Essential oil composition of Pterospartum tridentatum grown in Portugal. Food Chem. 2007, 102, 1083–1088. [Google Scholar] [CrossRef]
- Coelho, M.T.; Gonçalves, J.C.; Alves, V.; Martins, M.M. Antioxidant activity and phenolic content of extracts from different Pterospartum tridentatum populations growing in Portugal. Procedia Food Sci. 2011, 1, 1454–1458. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.L.S.; Rabe, T.; McGaw, L.J.; Jäger, A.K.; van Staden, J. Towards the scientific validation of traditional medicinal plants. Plant. Growth Reg. 2001, 34, 23–37. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Gil, C.; Duarte, A.P. Antioxidant activity of extracts of Portuguese shrubs: Pterospartum tridentatum, Cytisus scoparius and Erica spp. J. Med. Plant. Res. 2009, 3, 886–893. [Google Scholar]
- Luís, Â.; Domingues, F.; Duarte, A.P. Bioactive compounds, RP-HPLC analysis of phenolics, and antioxidant activity of some Portuguese shrub species extracts. Nat. Prod. Commun. 2011, 6, 1863–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Influence of the drying method in the antioxidant potential and chemical composition of four shrubby flowering plants from the tribe Genisteae (Fabaceae). Food Chem. Toxicol. 2011, 48, 2983–2989. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Estévez, J.C.; Silva-Pando, F.J. Antioxidant activity, total phenolic content and skin care properties of 35 selected plants from Galicia (NW Spain). Front. Life Sci. 2012, 6, 77–86. [Google Scholar] [CrossRef]
- Ferreira, F.M.; Dinis, L.T.; Azedo, P.; Galhano, C.I.C.; Simões, A.; Cardoso, S.M.; Domingues, M.R.M.; Pereira, O.R.; Palmeira, C.M.; Peixoto, F.P. Antioxidant capacity and toxicological evaluation of Pterospartum tridentatum flower extracts. CyTA J. Food 2012, 10, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Pterospartum tridentatum, Gomphrena globosa and Cymbopogon citratus: A phytochemical study focused on antioxidant compounds. Food Res. Int. 2014, 62, 684–693. [Google Scholar] [CrossRef] [Green Version]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Scientific validation of synergistic antioxidant effects in commercialized mixtures of Cymbopogon citratus and Pterospartum tridentatum or Gomphrena globose for infusions preparation. Food Chem. 2015, 185, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Caleja, C.; Finimundy, T.C.; Pereira, C.; Barros, L.; Calhelha, R.C.; Sokovic, M.; Ivanov, M.; Carvalho, A.M.; Rosa, E.; Ferreira, I.C.F.R. Challenges of traditional herbal teas: Plant infusions and their mixtures with bioactive properties. Food Funct. 2019, 10, 5939–5951. [Google Scholar] [CrossRef] [Green Version]
- Martins, N.; Ferreira, I.C.F.R.; Barros, L.; Carvalho, A.M.; Henriques, M.; Silva, S. Plants used in folk medicine: The potential of their hydromethanolic extracts against Candida species. Ind. Crops Prod. 2015, 66, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Aires, A.; Marrinhas, E.; Carvalho, R.; Dias, C.; Saavedra, M.J. Phytochemical composition and antibacterial activity of hydroalcoholic extracts of Pterospartum tridentatum and Mentha pulegium against Staphylococcus aureus isolates. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Martins, V.M.R.; Simões, J.; Ferreira, I.; Cruz, M.T.; Domingues, M.R.; Coimbra, M.A. In vitro macrophage nitric oxide production by Pterospartum tridentatum (L.) Willk. inflorescence polysaccharides. Carbohydr. Polym. 2017, 157, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Pinto, D.C.G.A.; Silva, A.M.S. Valorisation of Portuguese natural resources. Phytochem. Rev. 2020. [Google Scholar] [CrossRef]
- Paulo, A.; Martins, S.; Branco, P.; Dias, T.; Borges, C.; Rodrigues, A.I.; Costa, M.C.; Teixeira, A.; Mota-Filipe, H. The opposing effects of the flavonoids isoquercitrin and sissotrin, isolated from Pterospartum tridentatum, on oral glucose tolerance in rats. Phytother. Res. 2008, 22, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.F.M.; Pinto, D.C.G.A.; Silva, A.M.S. Chromones: A promising ring-system for new anti-inflammatory drugs. ChemMedChem 2016, 11, 2252–2260. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Gutiérrez-Venegas, G. Flavonoids exert multiple periodontic benefits including anti-inflammatory, periodontal ligament-supporting, and alveolar bone-preserving effects. Life Sci. 2018, 209, 435–454. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.W.; Murugain, D.; Leong, X.-F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: A mini review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef] [Green Version]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
- Klayciyan, A.; Orawa, H.; Fimmel, S.; Perschel, F.H.; González, J.-B.; Fitzner, R.G.; Orfanos, C.E.; Zouboulis, C.C. Nicotine and biochanin A, but not cigarette smoke, induce anti-inflammatory effects on keratinocytes and endothelial cells in patients with Behçet’s disease. J. Investig. Dermatol. 2007, 127, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Ming, X.; Ding, M.; Zhai, B.; Xiao, L.; Piao, T.; Liu, M. Biochanin A inhibits lipopolysaccharide-induced inflammation in human umbilical vein endothelial cells. Life Sci. 2015, 136, 36–41. [Google Scholar] [CrossRef]
- Wang, W.; Tang, L.; Li, Y.; Wang, Y. Biochanin A protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses. J. Neurol. Sci. 2015, 348, 121–125. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Wu, Y.-Y.; Huang, H.; He, C.; Li, W.-Z.; Wang, H.-L.; Chen, H.-Q.; Yin, Y.-Y. Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells. Int. J. Mol. Med. 2015, 35, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, W. Biochanin A inhibits lipopolysaccharide-induced inflammatory cytokines and mediators production in BV2 microglia. Neurochem. Res. 2015, 40, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, T.; Liu, X.; Cai, L.; Qi, J.; Zhang, P.; Li, Y. Biochanin A protects lipopolysaccharide/D-galactosamine-induced acute liver injury in mice by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Int. Immunopharmacol. 2016, 38, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-S.; Cho, I.-A.; Kang, K.-R.; You, J.-S.; Yu, S.-J.; Lee, G.-J.; Seo, Y.-S.; Kim, C.S.; Kim, D.K.; Kim, S.-G.; et al. Biochanin-A antagonizes the interleukin-1b-induced catabolic inflammation through the modulation of NFκB cellular signaling in primary rat chondrocytes. Biochem. Biophys. Res. Commun. 2016, 477, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, W.-Y.; Huang, H.; Li, W.-Z.; Chen, H.-Q.; Yin, Y.-Y. Biochanin A protects against lipopolysaccharide-induced damage of dopaminergic neurons both in vivo and in vitro via inhibition of microglial activation. Neurotox. Res. 2016, 30, 486–498. [Google Scholar] [CrossRef]
- Suliman, F.A.; Khodeer, D.M.; Ibrahiem, A.; Mehanna, E.T.; El-Kherbetawy, M.K.; Mohmmad, H.M.F.; Zaitone, S.A.; Moustafa, Y.M. Renoprotective effect of the isoflavonoid biochanin A against cisplatin induced acute kidney injury in mice: Effect on inflammatory burden and p53 apoptosis. Int. Immunopharmacol. 2018, 61, 8–19. [Google Scholar] [CrossRef]
- Alauddin; Chaturvedi, S.; Malik, M.Y.; Azmi, L.; Shukla, I.; Naseem, Z.; Rao, C.V.; Agarwal, N.K. Formononetin and biochanin A protects against ritonavir induced hepatotoxicity via modulation of NfκB/pAkt signaling molecules. Life Sci. 2018, 213, 174–182. [Google Scholar] [CrossRef]
- Bai, Y.; Li, Z.; Liu, W.; Gao, D.; Liu, M.; Zhang, P. Biochanin A attenuates myocardial ischemia/reperfusion injury through the TLR4/NF-κB/NLRP3 signaling pathway. Acta Cir. Bras. 2019, 34, e201901104. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Qin, H.; Li, Y.; Li, J.; Fu, L.; Li, M.; Jiang, C.; Yun, J.; Liu, Z.; Feng, Y.; et al. Biochanin A protect against lipopolysaccharide-induced acute injury in mice by regulating TLR4//NF-κB and PPAR-γ parhway. Microb. Pathogen. 2020, 138, 103846. [Google Scholar] [CrossRef]
- Kole, L.; Giri, B.; Manna, S.K.; Pal, B.; Ghosh, S. Biochanin A, an isoflavone, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur. J. Pharmacol. 2011, 653, 8–15. [Google Scholar] [CrossRef]
- Breikaa, R.M.; Algandaby, M.M.; El-Demerdas, E.; Abdel-Naim, A.B. Biochanin A protects against acute carbon tetrachloride-induced hepatotoxicity in rats. Biosci. Biotechnol. Biochem. 2013, 77, 909–916. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Ye, Z.; Zhuang, Z.; Gao, Y.; Tang, C.; Zhou, C.; Wang, C.; Zhang, X.; Xie, G.; Liu, J.; et al. Biochanin A reduces inflammatory injury and neuronal apoptosis following subarachnoid hemorrhage via suppression of the TLRs/TIRAP/MyD88/NF-κB pathway. Behav. Neurol. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piegholdt, S.; Pallauf, K.; Esatbeyoglu, T.; Speck, N.; Reiss, K.; Ruddigkeit, L.; Stocker, A.; Huebbe, P.; Rimbach, G. Biochanin A and prunetin improve epithelial barrier function in intestinal CaCo-2 cells via downregulation of ERK, NF-κB, and tyrosine phosphorylation. Free Radic. Biol. Med. 2014, 70, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Lin, B.; Lin, Z.; Lin, Y.; Lin, M.; Yang, X. Biochanin A ameliorates the cytokine secretion profile of lipopolysaccharide-stimulated macrophages by a PPARγ-dependent pathway. Mol. Med. Repor. 2012, 5, 217–222. [Google Scholar]
- Guo, M.; Lu, H.; Qin, J.; Qu, S.; Wang, W.; Guo, Y.; Liao, W.; Song, M.; Chen, J.; Wang, Y. Biochanin A provides neuroprotection against cerebral ischemia/reperfusion injury by Nrf2-mediated inhibition of oxidative stress and inflammation signaling pathway in rats. Med. Sci. Monit. 2019, 25, 8975–8983. [Google Scholar] [CrossRef]
- Yang, G.; Ham, I.; Choi, H.-Y. Anti-inflammatory effect of prunetin via suppression of NF-κB pathway. Food Chem. Toxicol. 2013, 58, 124–132. [Google Scholar] [CrossRef]
- Hu, H.; Li, H. Prunetin inhibits lipopolysaccharide -induced inflammatory cytokine production and MUC5AC expression by inactivating the TLR4/MyD88 pathway in human nasal epithelial cells. Biomed. Pharmacother. 2018, 106, 1469–1477. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Bai, Y.-Q.; Qi, M. Daidzein attenuates abdominal aortic aneurysm through NF-κB, p38MAPK and TGF-β1 pathways. Mol. Med. Rep. 2016, 14, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Atiq, A.; Shal, B.; Naveed, M.; Khan, A.; Ali, J.; Zeeshan, S.; Al-Sharari, S.D.; Kim, Y.S.; Khan, S. Diadzein ameliorates 5-fluorouracil-induced intestinal mucositis by suppressing oxidative stress and inflammatory mediators in rodents. Eur. J. Pharmacol. 2019, 843, 292–306. [Google Scholar] [CrossRef]
- Tomar, A.; Kaushik, S.; Khan, S.I.; Bisht, K.; Nag, C.N.; Arya, D.S.; Bhatia, J. The dietary isoflavone daidzein mitigates oxidative stress, apoptosis, and inflammation in CDDP-induced kidney injury in rats: Impact on the MAPK signaling pathway. J. Biochem. Mol. Toxicol. 2020, 34, e22431. [Google Scholar] [CrossRef]
- Zhang, F.; Ru, N.; Shang, X.-H.; Chen, J.-F.; Yan, C.; Li, Y.; Liang, J. Daidzein ameliorates spinal cord ischemia/reperfusion injury-induced neurological function deficits in Sprague-Dawley rats through PI3K/Akt signaling pathway. Exp. Ther. Med. 2017, 14, 4878–4886. [Google Scholar] [CrossRef]
- Han, S.; Wu, H.; Li, W.; Gao, P. Protective effects of genistein in homocysteine-induced endothelial cell inflammatory injury. Mol. Cell Biochem. 2015, 403, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.F.; Saleh, D.O.; Mostafa, R.E. Genistein ameliorates cyclophosphamide-induced hepatotoxicity by modulation of oxidative stress and inflammatory mediators. Open Access Maced. J. Med. Sci. 2017, 5, 836–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Z.-R.; Feng, X.-Q.; Li, N.; Qu, J.-X.; Feng, L.; Chen, L.; Chen, W.-F. G protein-coupled estrogen receptor is involved in the anti-inflammatory effects of genistein in microglia. Phytomedicine 2018, 43, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wei, J.; Lu, C.; Chen, H.; Zhong, X.; Lu, Y.; Li, L.; Huang, H.; Dai, Z.; Han, L. Genistein suppresses psoriasis-related inflammation through a STAT3-NF-κB-dependent mechanism in keratinocytes. Int. Immunopharmacol. 2019, 69, 270–278. [Google Scholar] [CrossRef]
- Chen, Y.; Le, T.H.; Du, Q.; Zhao, Z.; Liu, Y.; Zou, J.; Hua, W.; Liu, C.; Zhu, Y. Genistein protects against DSS-induced colitis by inhibiting NLRP3 inflammasome via TGR5-cAMP signaling. Int. Immunopharmacol. 2019, 71, 144–154. [Google Scholar] [CrossRef]
- Gan, M.; Shen, L.; Fan, Y.; Tan, Y.; Zheng, T.; Tang, G.; Niu, L.; Zhao, Y.; Chen, L.; Jiang, D.; et al. MicroRNA-451 and genistein ameliorate nonalcoholic steatohepatitis in mice. Int. J. Mol. Sci. 2019, 20, 6084. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Lv, J.; Jiang, N.; Wang, H.; Huang, H.; Zhang, L.; Li, S.; Zhang, N.; Fan, B.; Liu, X.; et al. Protective effects of genistein on the cognitive deficits induced by chronic sleep deprivation. Phytother. Res. 2020, 34, 846–858. [Google Scholar] [CrossRef]
- Bhattarai, G.; Poudel, S.B.; Kook, S.-H.; Lee, J.-C. Anti-inflammatory, anti-osteoclastic, and antioxidant activities of genistein protect against alveolar bone loss and periodontal tissue degradation in a mouse model of periodontitis. J. Biomed. Mat. Res. 2017, 195A, 2510–2521. [Google Scholar] [CrossRef]
- Pummoung, S.; Werawatganon, D.; Klaikeaw, N.; Siriviriyakul, P. Genistein-attenuated hepatic steatosis and inflammation in nonalcoholic steatohepatitis with bilateral ovariectomized rats. Pharmacogn. Mag. 2018, 14, S20–S24. [Google Scholar]
- Xu, L.; Liu, J.; Li, K.; Wang, S.; Xu, S. Genistein inhibits ang II- induced CRP and MMP-9 generations via Er-p38/ERK1/2-PPARγ-NF-κB signaling pathway in rat vascular smooth muscle cells. Life Sci. 2019, 216, 140–146. [Google Scholar] [CrossRef]
- Yoo, H.; Ku, S.-K.; Baek, Y.-D.; Bae, J.-S. Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflamm. Res. 2014, 63, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Khajevand-Khazaei, M.-R.; Mohseni-Moghaddam, P.; Hosseini, M.; Gholami, L.; Baluchnejadmojarad, T.; Roghani, M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57Bl/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT1. Eur. J. Pharmacol. 2018, 833, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Nkpaa, K.W.; Onyeso, G.I. Rutin attenuates neurobehavioral deficits, oxidative stress, neuroinflammation and apoptosis in fluoride treated rats. Neurosci. Lett. 2018, 682, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Kandemir, F.M.; Yildirim, S.; Kucukler, S.; Eser, G. Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J. Trace Elem. Med. Biol. 2019, 54, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Kandemir, F.M.; Darendelioglu, E.; Yildirim, S.; Kucukler, S.; Dortbudak, M.B. Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J. Trace Elem. Med. Biol. 2019, 56, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, B.A.; Adineh, M.; Hajiali, F.; Nassiri-Asl, M. Treatment with rutin—A therapeutic strategy for neutrophil-mediated inflammatory and autoimmune diseases. J. Pharmacopunct. 2017, 20, 52–56. [Google Scholar]
- Cai, C.; Liu, C.; Zhao, L.; Liu, H.; Li, W.; Guan, H.; Zhao, L.; Xiao, J. Effects of taxifolin on osteoclastogenesis in vitro and in vivo. Front. Pharmacol. 2018, 9, 1286. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-Q.; Wang, Y.-J.; Yang, G.-T.; Gao, Q.-L.; Tang, M.-X. Taxifolin inhibits receptor activator of NF-kB ligand-induced osteoclastogenesis of Human bone marrow-derived macrophages in vitro and prevents lipopolysaccharide-induced bone loss in vivo. Pharmacology 2019, 103, 101–109. [Google Scholar] [CrossRef]
- Pan, S.; Zhao, X.; Ji, N.; Shao, C.; Fu, B.; Zhang, Z.; Wang, R.; Qiu, Y.; Jin, M.; Kong, D. Inhibitory effect of taxifolin on mast cell activation and mast cell-mediated allergic inflammatory response. Int. Immunopharmacol. 2019, 71, 205–214. [Google Scholar] [CrossRef]
- Hu, C.; Ye, J.; Zhao, L.; Li, X.; Wang, Y.; Liu, X.; Pan, L.; You, L.; Chen, L.; Jia, Y.; et al. 5,7,3′,4′-Flavan-on-ol (taxifolin) protects against acetaminophen-induced liver injury by regulating the glutathione pathway. Life Sci. 2019, 236, 116939. [Google Scholar] [CrossRef] [PubMed]
- King, F.E.; Jurd, L. The chemistry of extractives from hardwoods. Part VIII. *the isolation of 5,4′-dihydroxy-7-methoxyisoflavone (prunetin) from the heartwood of Pterocarpus angolensis and a synthesis of 7,4′dihydroxy-5-methoxyisoflavone hitherto known as prunutsetin. J. Chem. Soc. 1952, 1952, 3190–3195. [Google Scholar]
- Siddiqui, M.T.; Siddiqi, M. Hypolipidemic principles of Cicer arietinum: Biochanin-A and formononetin. Lipids 1976, 11, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Raheja, S.; Girdhar, A.; Lather, V.; Pandita, D. Biochanin A: A phytoestrogen with therapeutic potential. Trends Food Sci. Technol. 2018, 79, 55–66. [Google Scholar] [CrossRef]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, S.; Kanwal, L.; et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907. [Google Scholar] [CrossRef] [PubMed]
- Liggins, J.; Bluck, L.J.C.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and genistein content of fruits and nuts. J. Nutr. Biochem. 2000, 11, 326–331. [Google Scholar] [CrossRef]
- Barlow, J.; Johnson, J.A.P.; Scofield, L. Fact Sheet on the Phytoestrogen Daidzein. BCERC COTC Fact. Sheet. 2007. Available online: https://www.zerobreastcancer.org/research/bcerc_factsheets_phytoestrogen_daidzein.pdf (accessed on 29 May 2020).
- Sun, M.-Y.; Ye, Y.; Xiao, L.; Rahman, K.; Xia, W.; Zhang, H. Daidzein: A review of pharmacological effects. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Barlow, J.; Johnson, J.A.P.; Scofield, L. Fact Sheet on the Phytoestrogen Genistein. BCERC COTC Fact. Sheet. 2007. Available online: https://www.zerobreastcancer.org/research/bcerc_factsheets_phytoestrogen_genistein.pdf (accessed on 29 May 2020).
- Kumar, M.; Singh, K.; Duraisamy, K.; Allam, A.A.; Ajarem, J.; Chow, B.K.C. Protective effect of genistein against compound 48/80 induced anaphylactoid shock via inhibiting MAS related G protein-coupled receptor Χ2 (MRGPRΧ2). Molecules 2020, 25, 1028. [Google Scholar] [CrossRef] [Green Version]
- Polkowski, K.; Mazurek, A.P. Biological properties of genistein. A review of in vitro and in vivo data. Acta Poloniae Pharm. Drug Res. 2000, 57, 135–155. [Google Scholar]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhabi, N.A.; Arasu, M.V.; Park, C.H.; Park, S.U. An up-to-date review of rutin and its biological and pharmacological activities. EXCLI J. 2015, 14, 59–63. [Google Scholar]
- Rauf, A.; Imran, M.; Patel, S.; Muzaffar, R.; Bawazeer, S.S. Rutin: Exploitation of the flavonol for health and homeostasis. Biomed. Pharmacother. 2017, 96, 1559–1561. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar]
- Riaz, H.; Raza, S.A.; Aslam, M.M.; Ahmad, M.S.; Ahmad, M.A.; Maria, P. An updated review of pharmacological, standardization methods and formulation development of rutin. J. Pure App. Microbiol. 2018, 12, 127–132. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Manzoni, A.G.; Passos, D.F.; Leitemperger, J.W.; Storck, T.R.; Doleski, P.H.; Jantsch, M.H.; Loro, V.L.; Leal, D.B.R. Hyperlipidemia-induced lipotoxicity and immune activation in rats are prevented by curcumin and rutin. Int. Immunopharmacol. 2020, 81, 106217. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Long. 2018, 2018. [Google Scholar] [CrossRef]
- Mazumder, M.K.; Borah, A.; Choudhury, S. Inhibitory potential of plant secondary metabolites on anti-Parkinsonian drug targets: Relevance to pathophysiology, and motor and non-motor behavioural abnormalities. Med. Hypotheses 2020, 137, 109544. [Google Scholar] [CrossRef]
- Harikrishnan, H.; Jantan, I.; Alagan, A.; Haque, M.A. Modulation of cell signaling pathways by Phyllanthus amarus and its major constituents: Potential role in the prevention and treatment of inflammation and cancer. Inflammopharmacology 2020, 28, 1–18. [Google Scholar] [CrossRef]
- Hasumura, M.; Yasuhara, K.; Tamura, T.; Imai, T.; Mitsumori, K.; Hirose, M. Evaluation of the toxicity of enzymatically decomposed rutin with 13-weeks dietary administration to Wistar rats. Food Chem. Toxicol. 2004, 42, 439–444. [Google Scholar] [CrossRef]
- Boyle, S.P.; Dobson, V.L.; Duthie, S.J.; Hinselwood, D.C.; Kyle, J.A.M.; Collins, A.R. Bioavailability and efficiency of rutin as an antioxidant: A human supplementation study. Eur. J. Clin. Nutr. 2000, 54, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Ragheb, S.R.; El Wakeel, L.M.; Nasr, M.S.; Sabri, N.A. Impact of rutin and vitamin C combination on oxidative stress and glycemic control in patients with type 2 diabetes. Clin. Nutr. ESPEN 2020, 35, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Das, M.K. Rutin-phospholipid complex in polymer matrix for long-term delivery of rutin via skin for treatment of inflammatory diseases. Artif. Cells NanoMed. Biothechnol. 2018, 46, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.B.; Bhalla, T.N.; Gupta, G.P.; Mitra, C.R.; Bhargava, K.P. Anti-inflammatory activity of taxifolin. Jpn. J. Pharmacol. 1971, 21, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef] [PubMed]






| Plant Part | Solvent | Activity Tested | Method | Ref. |
|---|---|---|---|---|
| Aerial parts | Ethanol and water | Antioxidant (ethanol, IC50 = 60.39 ± 1.79 μg/mL; water, IC50 = 42.97 ± 1.69 μg/mL) | DPPH scavenging β-Carotene bleaching test | [24] |
| Flowers, stems and leaves | Methanol | Antioxidant (flowers, IC50 = 26.1 ± 1.3 mg/L; stems and leaves, IC50 = 69.7 ± 11.9 mg/L) | DPPH scavenging β-Carotene bleaching test | [25] |
| Flowers | Methanol | Antioxidant | DPPH scavenging (IC50 = 0.15 ± 0.01 mg/mL) β-Carotene bleaching test (IC50 = 0.14 ± 0.02 mg/mL) Reducing power (IC50 = 0.13 ± 0.00 mg/mL) TBARS inhibition (IC50 = 0.12 ± 0.02 mg/mL) | [26] |
| Flowers and leaves | Hydroethanolic | Antioxidant (flowers, IC50 = 1016 mg/L; leaves, IC50 = 704 mg/L | DPPH scavenging β-Carotene bleaching test Reducing power ABTS scavenging | [27] |
| Purchased plant material | Water | Antioxidant (%AA = 169.5 ± 17.2) | β-Carotene bleaching test ABTS scavenging | [28] |
| Purchased plant material | Methanol | Antioxidant | DPPH scavenging (IC50 = 0.18 ± 0.01 mg/mL) β-Carotene bleaching test (IC50 = 0.48 ± 0.09 mg/mL) Reducing power (IC50 = 0.11 ± 0.00 mg/mL) TBARS inhibition (IC50 = 1.18 ± 0.06 mg/mL) | [29] |
| Purchased plant material | Hot water | Antioxidant | DPPH scavenging (IC50 = 50 ± 1 μg/mL) β-Carotene bleaching test (IC50 = 266 ± 25 μg/mL) Reducing power (IC50 = 105 ± 2 μg/mL) TBARS inhibition (IC50 = 93 ± 4 μg/mL) | [30] |
| Flowers | Hot water | Antioxidant (TABARS, IC50 = 8.4 ± 0.2 μg/mL; OxHLIA, IC50 = 37.7 ± 0.9 μg/mL) | TBARS inhibition Oxidative haemolysis inhibition | [31] |
| Flowers | Hydromethanolic | Antifungal (Candida albicans, 10 mm inhibition zone; Candida glabrata, 11 mm inhibition zone) | Disc diffusion test | [32] |
| Aerial parts | Hydromethanolic | Antibacterial (Staphylococcus aureus, MIC = 39.1 μg/mL) | Microplate bioassay | [33] |
| Flowers | Hot water | Antimicrobial (Escherichia coli, MIC = 0.5 mg/mL; Salmonela typhimurium, MIC = 1 mg/mL; Bacillus cereus, MIC = 1 mg/mL; Listeria monocytogenes, MIC = 1 mg/mL; Aspergillus niger, MIC = 8 mg/mL; Aspergillus versicolor, MIC = 0.5 mg/mL; Penicillium funiculosum, MIC = 0.5 mg/mL; Penicillium verrucosum, MIC = 0.5 mg/mL) | Disc diffusion test | [31] |
| Flowers | Hot water | Cytotoxicity (HeLa, GI50 = 242 ± 10 μg/mL; HepG2, GI50 = 262 ± 11 μg/mL) | Against tumor cells HeLa, HepG2, MCF-7 and NCi-H460 and non-tumor cells PLP2 | [31] |
| Inflorescences | Hot water | Immunostimulatory (significant activity for 200 μg/mL) | Macrophage cell viability and NO production | [34] |
| Purchased plant material | Water | Toxicity (non toxic at 375 mg/L) | MTT assay; mitochondrial swelling, | [28] |
| Flowers, leaves, stems and roots | Ethanol | Toxicity (non toxic at 100 μg/mL) | Resazurin assay | [8] |
| Flowers | Hot water | Anti-inflammatory (>400 μg/mL) | Determination of LPS-induced NO production by Murine macrophage (RAW 264.7) cell lines | [31] |
| Flowers, leaves, stems and roots | Ethanol | Anti-inflammatory (significantat 100 μg/mL) | LPS-induced transcription of pro-inflammatory genes IL-1β, Nos2, Ptgs2, IL-6, and TNF-α; Western blot analysis | [8,35] |
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nº | Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 | Ref. |
| 1a | Sissotrin | H | OGlc | H | OH | H | OMe | H | [8,20,29,30,36] |
| 1b | Genistin | H | OGlc | H | OH | H | OH | H | [20,29,30,33,36] |
| 1c | 5,5′-Dihydroxy-3′-metoxi- -isoflavone-7-O-β-glucoside | H | OGlc | H | OH | OMe | H | OH | [8,20,29,30,31,36] |
| 1d | Prunetin | H | OMe | H | OH | H | OH | H | [8,20,29,30,36] |
| 1e | Genistein | H | OH | H | OH | H | OH | H | [8,27,29,30,31,33,36] |
| 1f | 7-Methylorobol | H | OMe | H | H | OH | OH | H | [29,30,36] |
| 1g | Genistein-8-C-glucoside | Glc | OH | H | OH | H | OH | H | [29,30,31] |
| 1h | Biochanin A | H | OH | H | OH | H | OMe | H | [8,29,30] |
| 1i | 5-Hydroxy-4′,7-dimethoxy- -isoflavone | H | OMe | H | OH | H | OMe | H | [8] |
| 1j | Daidzein | H | OH | H | H | H | OH | H | [8] |
| 2a | Luteolin-O-glucuronide | H | OGlc | H | OH | OH | OH | H | [28] |
| 2b | Luteolin-O-(O-acetyl)glucuronide | H | OGlcA-Ac | H | OH | OH | OH | H | [28] |
| 2c | Apigenin | H | OH | H | OH | H | OH | H | [33] |
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nº | Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Ref. |
| 3a | Isoquercitrin | Glc | H | OH | H | OH | OH | OH | H | [20,29,30,31,33,36] |
| 3b | Myricetin-6-C-glucoside | H | H | OH | Glc | OH | H | OH | OH | [8,29,30,36] |
| 3c | Rutin | Rha-Glc | H | OH | H | OH | OH | OH | H | [29,30,31,33,36] |
| 3d | Isorhamnetin-O-glucoside | Glc | H | OH | H | OH | OMe | OH | H | [28] |
| 3e | Myricetin-3,4′-di-O- -glucoside | Glc | H | OH | H | OH | OH | OGlc | OH | [28] |
| 3f | Astragalin | Glc | H | OH | H | OH | H | OH | H | [8] |
| 3g | Isorhamnetin-3-O- -glucoside | Glc | H | OH | H | OH | OMe | OH | H | [8] |
| 3h | Kaempferol | H | H | OH | H | OH | H | OH | H | [8] |
| Flavonoid | Model | Mechanisms |
|---|---|---|
| Biochanin A | In vitro: cytokine release from keratinocytes and HMEC-1 endothelial cells in serum from patients with Behçet’s disease [41] In vitro: LPS-induced inflammation in HUVED cells [42] In vivo: focal cerebral ischemia–reperfusion model [43] In vitro: LPS-induced pro-inflammatory responses in murine BV2 microglial cells [44] In vitro: LPS-induced inflammatory cytokines and mediators production in murine BV2 microglial cells [45] In vivo: LPS/GalN-induced liver injury [46] Ex vivo: interleukin-1β-induced catabolic inflammation through the modulation of NFκB cellular signaling in primary rat chondrocytes [47] In vitro and in vivo: LPS-induced damage of dopaminergic neurons [48] In vivo: cisplatin induced acute kidney injury in mice [49] In vivo: ritonavir induced hepatotoxicity [50] In vivo: transient coronary ligation in Sprague-Dawley rats [51] In vivo: LPS-induced acute lung injury in mice [52] In vitro: LPS-induced NO production, LPS-induced IKK activity, LPS-induced phosphorylation of IκBα and p38 MAPK [53] In vitro: CCl4-induced hepatotoxicity in rats [54] In vivo: Sprague-Dawley rat subarachnoid hemorrhage [55] In vitro: barrier function of intestinal epithelial CaCo-2/TC-7 cells via TEER measurements [56] In vitro: LPS-stimulated macrophages [57] In vivo: focal cerebral ischemia established by middle cerebral artery occlusion [58] | ↓IL-8 ↓IL-8, TNF-α, VCAM-1, ICAM-1, E-selection ↑PPAR-γ ↓IL-8, TNF-α, P38 expression ↓IL-1β, TNF-α, NO, phosphorylation of JNK, ERK and p38 ↓IL-1β, TNF-α, NO, PGE2, NF-κB ↑PPAR-γ IL-1β, TNF-α, ALT, AST, MDA, TXNIP, NLRP3 inflammasome ↑SOD, GPx, catalase, HO-1, Nrf2 ↓IL-1β, TNF-α, IL-6, IL-1α, INFγ, IL- 2, GM-CSF, fractalkine, MCP-1, MIP-3α, LIX ↓IL-1β, TNF-α, IL-6, phosphorylation of JNK, ERK and p38, ↓IL-1β, TNF-α, caspase-3, p53 protein ↓IL-1β, IL-6 ↑IL-10 ↓IL-1β, IL-18, IL-6, TNF-α IL-1β, IL-6, TNF-α, TLR4/NF-κB ↑PPAR-γ IL-6, TNF-α PPAR-γ, PPAR-α iNOS, COX2, TNF-α sTNFR1, TNF-α, NF-κB, ERK, tyrosine phosphorylation ↑SOD, GSH-Px, HO-1, Nrf2 ↓iNOS, phosphorylation of IκBα and p38 MAPK ↓TLR/NF-κB |
| Prunetin | In vitro: barrier function of intestinal epithelial CaCo-2/TC-7 cells via TEER measurements [56] In vitro: LPS-stimulated RAW 264.7 macrophage [59] In vivo: LPS-induced septic shock [59] In vitro: LPS-induced in- flammatory response and MUC5AC expression [60] | ↓sTNFR1, TNF-α, NF-κB, ERK, tyrosine phosphorylation ↓iNOS, PGE2, COX2, NF-κB, p38, IL-1β, TNF-α IL-1β, TNF-α IL-8, IL-6, MUC5AC, TLR4/MyD88 |
| Daidzein | In vitro: LPS-stimulated macrophages [57] In vivo: angiotensin II-induced AAA [61] In vivo: 5-fluorouracil-induced intestinal mucositis [62] In vivo: cisplatin-induced kidney injury [63] In vivo: ischemia/reperfusion injury-induced neurological function deficits in Sprague-Dawley [64] | ↓IL-6 ↓IL-1β, TNF-α, NF-κB, iNOS, COX-2, p38MAPK, TGF-β1 ↓IL-1β, IL-6, TNF-α, NO, COX-2 ↓IL-6, TNF-α, MDA, NO, COX-2, MAPK ↑SOD, GSH ↓TNF-α, NF-κB subunit p65 |
| Genistein | In vitro: LPS-stimulated macrophages [57] In vitro: homocysteine-induced endothelial cell inflammation [65] In vivo: cyclophosphamide - induced hepatotoxicity [66] In vivo: LPS-induced microglial activation in murine BV2 microglial cell line and primary microglial culture [67] In vivo: imiquimod- induced psoriasis-like lesions in mice [68] In vivo: DSS-induced murine colitis [69] In vivo: NASH mouse model [70] In vivo: chronic sleep deprivation [71] In vitro: barrier function of intestinal epithelial CaCo-2/TC-7 cells via TEER measurements [56] In vivo: mouse model of periodontitis [72] In vivo: high-fat high-fructose diet-induced NASH rats [73] In vitro: angiotensin II-stimulated CRP and MMP-9 expression in VSMC [74] | ↓IL-6, TNF-α PPAR-γ, PPAR-α NF-κB subunit p65, IL-6, ICAM-1 ↓IL-1β, COX-2, MPO ↓IL-1β, IL-6, COX-2, iNOS, TNF-α, NF-κB, MAPK ↓IL-1β, IL-6, IL-8, TNF-α, IL-17, IL-23, CCL2, NF-κB, VEGFA ↓IL-1β, IL-18, TNF-α, MPO, NLRP3 inflammasome ↓IL-6, TNF-α, ↓IL-1β, IL-6, COX-2, iNOS, TNF-α, NF-κB p65 ↑HO-1, Nrf2 ↓sTNFR1, tyrosine phosphorylation ↓TNF-α, COX-2, Nos2, ICAM-1, MMP-2, MMP-9 ↓TNF-α, NF-κB ↓p-ERK1/2, p-p38, NF-κB ↑PPAR-γ, |
| Rutin | In vivo: HMGB1-induced inflammation and CLP-induced sepsis model [75] In vivo: LPS-induced acute endotoxemic kidney injury in C57BL/6 mice [76] In vivo: NaF-induced neurotoxicity [77] In vivo: HgCl2-induced nephrotoxicity [78] In vivo: HgCl2-induced hepatotoxicity [79] In vitro: PMA-induced neutrophil stimulation [80] | ↓TLR 4, RAGE, p38 MAPK, VCAM-1, ICAM-1, ERK1/2, NF-κB ↓TLR 4, COX-2, TNF-α, IL-6, SIRT1, NF-κB ↓IL-1β, IL-6, TNF-α ↓IL-1β, IL-33, TNF-α, NF-κB, Bcl-3 ↓IL-1β, TNF-α, NF-κB, Bcl-3, Bcl-2, Bax, p53, p38 MAPK, caspase-3 ↓NO, TNF-α, MPO |
| Taxifolin | In vitro: osteoclastogenesis [81] In vivo: and ovariectomy-induced osteoporosis [81] In vivo: osteolysis model [82] In vitro: on IgE/Ag-stimulated mast cells including BMMCs [83] In vivo: acetaminophen-induced liver injury [84] | ↓AKT, RANKL ↓TNF-α, IL-1β, NF-κB, MAPK, NFATc1, MMP-9, cathepsin K, TRAP ↓MAPK, p38, ERK, JNK; RANKL, NF-κB ↓LTC4, IL-6, COX-2, TNF-α, NF-κB ↓ inhibiting metabolic activation mediated by CYP450 enzymes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, D.C.G.A.; Simões, M.A.M.; Silva, A.M.S. Genista tridentata L.: A Rich Source of Flavonoids with Anti-Inflammatory Activity. Medicines 2020, 7, 31. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines7060031
Pinto DCGA, Simões MAM, Silva AMS. Genista tridentata L.: A Rich Source of Flavonoids with Anti-Inflammatory Activity. Medicines. 2020; 7(6):31. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines7060031
Chicago/Turabian StylePinto, Diana C. G. A., Mark A. M. Simões, and Artur M. S. Silva. 2020. "Genista tridentata L.: A Rich Source of Flavonoids with Anti-Inflammatory Activity" Medicines 7, no. 6: 31. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines7060031