Characteristics of Black Ginseng (Panax ginseng C.A. Mayer) Production Using Ginseng Stored at Low Temperature after Harvest
Abstract
:1. Introduction
2. Results
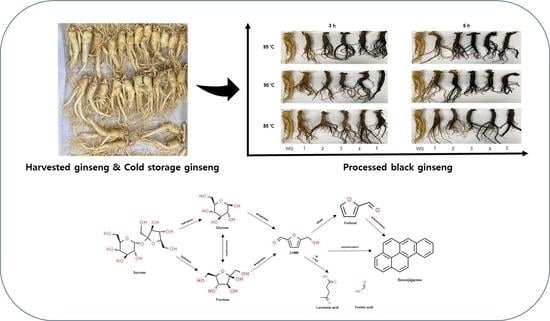
2.1. Steam Processing and Appearance Observations
2.2. Free Sugar
2.3. Benzo[a]pyrene
3. Materials and Methods
3.1. Materials and Equipment
3.2. Steam Processing and Appearance Observation
3.3. Sample Preparation
3.3.1. Benzo[a]pyrene
3.3.2. Free Sugar
3.4. Analysis of Benzo[a]pyrene by HPLC-FLD
3.5. Analysis of Free Sugar by HPLC-RID
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Wang, D.; Ru, W.; Qin, Y.; Zhou, X. An overview on ginseng and energy metabolism. In Sustained Energy for Enhanced Human Functions and Activity; Bagchi, D., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 205–229. [Google Scholar]
- Xue, Q.; He, N.; Wang, Z.; Fu, X.; Aung, L.H.H.; Liu, Y.; Li, M.; Cho, J.Y.; Yang, Y.; Yu, T. Functional roles and mechanisms of ginsenosides from Panax ginseng in atherosclerosis. J. Ginseng Res. 2021, 45, 22–31. [Google Scholar]
- Chang, J.-K.; Park, C.-K.; Shim, K.-H. Chang in chemical components of red ginseng processed from the fresh ginseng stored at low temperature. Korean J. Food Preserv. 2003, 10, 158–161. [Google Scholar]
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Kyung, J.S.; Lee, Y.Y.; In, G.; Park, C.-K.; Han, C.-K. Physiological and pharmacological features of the non-saponin components in Korean Red Ginseng. J. Ginseng Res. 2020, 44, 527–537. [Google Scholar] [CrossRef]
- Lee, J.W.; Ji, S.-H.; Choi, B.-R.; Lee, Y.-G.; Kim, H.-G.; Kim, G.-S.; Kim, K.; Lee, Y.-H.; Baek, N.-I.; Lee, D.Y. UPLC-QTOF/MS-based metabolomics applied for the quality evaluation of four processed panax ginseng products. Molecules 2018, 23, 2062. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Shen, G.N.; Kim, E.K.; Shin, H.J.; Myung, C.S.; Oh, H.J.; Kim, D.H.; Roh, S.S.; Cho, W.; Seo, Y.B.; et al. Preparation of black ginseng and its antitumor activity. Korean J. Oriental Physiol. Pathol. 2006, 20, 951. [Google Scholar]
- Song, G.Y.; Roh, S.S.; Seo, Y.B.; Park, Y.J.; Myung, C.S. Effect of Black Ginseng on Body Weight and Lipid Profiles. Yakhak Hoeji 2006, 50, 381. [Google Scholar]
- Park, J.Y.; Lee, D.-S.; Kim, C.-E.; Shin, M.-S.; Seo, C.-S.; Shin, H.-K.; Hwang, G.S.; An, J.M.; Kim, S.-N.; Kang, K.S. Effects of fermented black ginseng on wound healing mediated by angiogenesis through the mitogen-activated protein kinase pathway in human umbilical vein endothelial cells. J. Ginseng Res. 2018, 42, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.M.; Lianlian, Z.; Luqi, H.; Deqiang, D. Black Ginseng and Its Saponins: Preparation, Phytochemistry and Pharmacological Effects. Molecules 2019, 24, 1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, E.-J.; Kang, S.-J.; Kim, A.-J. Effects of Steam-And Dry-Processing Temperatures on the Benzo(a)pyrene Content of Black and Red Ginseng. Korean J. Food Nutr. 2009, 22, 199–204. [Google Scholar]
- Cho, Y.H.; Song, K.Y.; Baek, M.-K.; Lee, J.-W.; Lee, G.-W. Study on Extraction Condition and Analysis Methods of Benzopyrene in Black Ginseng. Yakhak. Hoeji 2012, 56, 145–151. [Google Scholar]
- Gelboin, H.V. Benzo[a]pyrene Metabolism, Activation, and Carcinogenesis: Role and Regulation of Mixed-Function Oxidases and Related Enzymes. Physiol. Rev. 1980, 60, 1107–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, K.Y.; Lee, N.R.; Moon, B.D.; Song, G.Y.; Shin, H.S.; Choi, J.E. Changes of Ginsenoside and Color from Black Ginseng Prepared by Steaming-Drying Cycles. Korean J. Med. Sci. 2012, 20, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Sin, K.; Na, Y.; Lee, J.; Cho, W. Control of Steaming Process for the Production of High Quality Red Ginseng. Korean Chem. Eng. Res. 2014, 52, 587–591. [Google Scholar]
- Do, J.-H.; Kim, S.-D.; Oh, H.-I.; Hong, S.-K. Effect of Sugars, Amino acids and Inorganic Nitrogenous Compounds on the Acceleration of Browning in Ginseng. J. Korean Agric. Chem. Soc. Sept. 1982, 25, 161–165. [Google Scholar]
- Jo, J.-S.; Oh, S.-K.; Cho, Y.-H.; Kim, H.-J.; Hwang, M.-H. Physicochemical Properties of Korean Ginseng (Panax ginseng, C.A Meyer) Root Polysaccharides–Change of physicochemical properties of the starch during storage and heat treatment. Korean J. Ginseng Sci. 1985, 9, 161–165. [Google Scholar]
- Yoon, D.H.; Shin, W.C.; Lee, Y.-S.; Kim, S.M.; Baek, M.-I.; Lee, D.Y. A Comparative Study on Processed Panax ginseng Products using HR-MAS NMR-Based Metabolomics. Molecules 2020, 25, 1390. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Do, J.H. Current Studies on Browning Reaction Products and Acidic Polysaccharide in Korean Red Ginseng. J. Ginseng Res. 2006, 30, 41–48. [Google Scholar]
- Yen, G.-C.; Tsai, L.-C.; Lii, J.-D. Antimutagenic Effect of Maillard Browning Products Obtained from Amino Acids and Sugars. Food Chem. Toxic. 1992, 30, 127–132. [Google Scholar] [CrossRef]
- Choi, J.E.; Nam, K.Y.; Li, X.; Kim, B.Y.; Cho, H.S.; Hwang, K.B. Changes of Chemical Compositions and Ginsenoside Contents of Different Root Parts of Ginsengs with Processing Method. Korean J. Med. Crop. Sci. 2010, 18, 118–125. [Google Scholar]
- Hong, H.-D.; Kim, Y.-C.; Rho, J.; Kim, K.-T.; Lee, Y.-C. Changes on Physicochemical Properites of Panax ginseng C.A Meyer during Repeated Steaming Process. J. Ginseng Res. 2007, 31, 222–229. [Google Scholar]
- Ko, S.-R.; Choi, K.-J.; Han, K.-W. Comparison of Proximate Composition, Mineral Nutrient, Amino Acid and Free Sugar Contents of Several Panax Species. Korean J. Ginseng Sci. 1996, 20, 36–41. [Google Scholar]
- Kim, H.-J.; Jo, J.-S.; Yoo, Y.-J. Physico Chemical Properties of Korean Ginseng (Panax ginseng CA. Meyer) Root Starch II. Chemical Properties of the Starch. Korean J. Ginseng Sci. 1984, 8, 124–134. [Google Scholar]
- Rhee, S.H.; Zong, M.S. A study on the Analysis of Amino Acid in Korean Ginseng. Korean J. Environ. Health Soc. 1983, 9, 37–53. [Google Scholar]
- Li, X.G. Studies on the Transforming Mechanism of Amino Acid Components in Ginseng in the Course of Ginseng Processing. Korean J. Ginseng Sci. 1992, 16, 64–67. [Google Scholar]
- Liu, Z.; Wen, X.; Wang, C.-Z.; Li, W.; Huang, W.-H.; Xia, J.; Ruanm, C.-C.; Yuan, C.S. Remarkable impact of amino acids on ginsenoside transformation from fresh ginseng to red ginseng. J. Ginseng Res. 2020, 44, 424–434. [Google Scholar] [CrossRef]
- Wilson, K.; Lee, A.F. Bio-based chemicals from biorefining: Carbohydrate conversion and utilization. In Advances in Biorefineries, 1st ed.; Woodhead Publishing: Waltham, MA, USA, 2014; pp. 624–658. [Google Scholar]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.-W.; Kim, H.-J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal Carbonization as a Valuable Tool for Energy and Environmental Applications. A Rev. Energ. 2020, 13, 4098. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Shin, J.-K.; Lee, S.-W.; Yoon, S.-R.; Chung, H.-S.; Jeong, Y.-J.; Choi, M.-S.; Lee, C.-M.; Moon, K.-D.; Kwon, J.-H. Quality and Functional Properties of Red Ginseng Prepared with Different Steaming Time and Drying Methods. Korean J. Food Sci. Technol. 2007, 39, 494–499. [Google Scholar]
- GINSENG INDUSTRY ACT. Agriculture, Food and Rural Affairs, Korea Ministry of Government Legislation. Act. No. 14769. 2017. Available online: https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=43270&type=sogan&key=7 (accessed on 10 February 2021).






| Steaming Process | Steaming Cycles | ||||||
|---|---|---|---|---|---|---|---|
| WG (2) | 1 | 2 | 3 | 4 | 5 | ||
| S-T95-3h (1) | ΔL | 68.24 ± 0.06 (3) | 36.30 ± 0.04 * | 31.53 ± 0.11 * | 30.44 ± 0.20 * | 28.46 ± 0.04 * | 23.52 ± 0.03 * |
| Δa | 8.16 ± 0.02 | 8.91 ± 0.02 | 6.73 ± 0.02 | 5.52 ± 0.01 | 3.08 ± 0.01 * | 2.10 ± 0.02 * | |
| Δb | 33.42 ± 0.04 | 22.77 ± 0.02 | 12.51 ± 0.03 | 8.55 ± 0.03 | 7.07 ± 0.01 | 5.26 ± 0.03 | |
| ΔE * | 76.42 ± 0.08 | 43.77 ± 0.04 | 34.58 ± 0.11 | 32.10 ± 0.21 | 29.49 ± 0.04 | 24.19 ± 0.05 | |
| S-T95-6h | ΔL | 68.24 ± 0.06 | 37.09 ± 0.12 * | 20.31 ± 0.14 * | 18.11 ± 0.18 * | 20.26 ± 0.02 * | 22.49 ± 0.06 * |
| Δa | 8.16 ± 0.02 | 9.81 ± 0.01 | 4.91 ± 0.04 | 1.81 ± 0.02 * | 1.18 ± 0.01 * | 1.68 ± 0.07 * | |
| Δb | 33.42 ± 0.04 | 21.09 ± 0.05 * | 6.85 ± 0.06 * | 1.26 ± 0.02 * | 3.13 ± 0.01 * | 5.24 ± 0.15 * | |
| ΔE | 76.42 ± 0.08 | 43.78 ± 0.13 | 21.98 ± 0.16 | 18.25 ± 0.18 | 20.53 ± 0.03 | 23.15 ± 0.18 | |
| H-T95-3h | ΔL | 52.63 ± 2.68 | 37.27 ± 1.07 * | 37.39 ± 0.62 * | 30.98 ± 0.04 * | 21.35 ± 0.23 * | 21.92 ± 0.12 * |
| Δa | 9.51 ± 2.02 | 6.19 ± 0.06 * | 5.54 ± 0.11 * | 7.51 ± 0.01 * | 3.43 ± 0.02 * | 3.90 ± 0.01 * | |
| Δb | 26.74 ± 3.85 | 23.51 ± 0.49 * | 17.95 ± 0.14 * | 15.41 ± 0.01 * | 7.90 ± 0.05 * | 7.04 ± 0.01 * | |
| ΔE | 59.84 ± 4.33 | 44.50 ± 1.64 | 41.84 ± 0.48 | 35.41 ± 0.04 | 23.02 ± 0.23 | 23.35 ± 0.11 | |
| H-T95-6h | ΔL | 52.63 ± 2.68 | 28.90 ± 0.14 * | 35.23 ± 0.36 * | 22.93 ± 0.40 * | 24.67 ± 0.36 * | 28.08 ± 0.16 * |
| Δa | 9.51 ± 2.02 | 6.16 ± 0.03 * | 7.45 ± 0.10 * | 3.61 ± 0.14 * | 3.99 ± 0.13 * | 7.77 ± 0.19 * | |
| Δb | 26.74 ± 3.85 | 17.45 ± 0.17 * | 19.12 ± 0.17 * | 7.30 ± 0.15 * | 8.30 ± 0.27 * | 10.80 ± 0.30 * | |
| ΔE | 59.84 ± 4.33 | 34.32 ± 0.13 | 40.78 ± 0.13 | 24.33 ± 0.34 | 26.33 ± 0.44 | 31.07 ± 0.29 | |
| Steaming Process | Steaming Times | Sucrose (mg/g) | Glucose (mg/g) | Fructose (mg/g) | Maltose (mg/g) |
|---|---|---|---|---|---|
| Control | S-WG (2) | (3) 381.11 ± 73.97 | N.D | N.D | N.D |
| M-WG | 308.45 ± 4.74 | N.D | 9.41 ± 4.03 | N.D | |
| S-T85-3h | 1 3 5 | 302.02 ± 35.69 261.88 ± 62.90 224.30 ± 8.95 | 8.81 ± 2.87 4.09 ± 3.25 17.22 ± 7.76 | 17.00 ± 5.48 25.24 ± 5.34 57.73 ± 9.63 | 3.01 ± 2.31 N.D N.D |
| S-T90-3h (1) | 1 | 301.55 ± 3.52 * | 2.24 ± 5.87 | 11.04 ± 1.82 * | 18.40 ± 5.64 |
| 3 | 258.35 ± 3.04 * | 5.06 ± 1.53 * | 37.07 ± 2.16 * | N.D | |
| 5 | 148.87 ± 4.85 * | 38.67 ± 2.80 * | 92.02 ± 1.63 * | N.D | |
| S-T95-3h | 1 | 283.05 ± 74.52 * | 15.17 ± 6.90 | 29.24 ± 8.64 * | 23.07 ± 3.95 * |
| 3 | 146.87 ± 5.23 * | 27.74 ± 11.81 * | 85.86 ± 1.53 * | 6.50 ± 11.72 * | |
| 5 | 23.49 ± 1.87 * | 9.20 ± 7.10 * | 72.86 ± 1.08 * | N.D | |
| S-T85-6h | 1 3 5 | 300.57 ± 17.82 197.90 ± 12.03 106.14±11.50 | 5.65 ± 2.04 6.01 ± 1.25 20.05 ± 7.89 | 14.45 ± 2.54 53.66 ± 8.62 85.13 ± 7.33 | 8.12 ± 3.69 N.D N.D |
| S-T90-6h | 1 | 301.68 ± 17.40 * | 2.08 ± 0.54 | 25.01 ± 8.16 * | N.D |
| 3 | 160.79 ± 8.79 * | 24.46 ± 8.06 * | 69.71 ± 4.61 * | N.D | |
| 5 | 27.06 ± 2.99 * | 12.65 ± 3.28 * | 96.56 ± 2.17 * | N.D | |
| S-T95-6h | 1 | 258.77 ± 6.28 * | 7.75 ± 6.88 | 25.78 ± 6.72 * | N.D |
| 3 | 46.17 ± 3.76 * | 14.50 ± 5.44 | 86.80 ± 5.76 * | N.D | |
| 5 | N.D | 35.10 ± 4.77 * | 88.26 ± 1.98 * | N.D | |
| H-T95-6h1) | 1 | 193.72 ± 60.92 * | 15.32 ± 5.09 | 27.11 ± 7.75 | 54.79 ± 14.64 * |
| 3 | 158.93 ± 51.37 * | 34.63 ± 12.75 | 59.03 ± 15.14 | 44.90 ± 13.84 * | |
| 5 | 35.79 ± 15.37 * | 63.22 ± 28.70 * | 106.72 ± 35.80 * | 33.15 ± 22.17 * |
| Steaming Process | Steaming Cycles | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| (1) S-T85-3h | (2) 0.50 ± 0.18 | 0.51 ± 0.17 | 0.51 ± 0.07 | 0.63 ± 0.12 | 0.58 ± 0.08 |
| S-T85-6h | 1.11 ± 0.57 | 4.80 ± 1.41 * | 4.63 ± 1.49 * | 5.31 ± 1.12 * | 5.17 ± 1.05 * |
| S-T90-3h | 0.61 ± 0.09 | 1.22 ± 0.27 * | 1.56 ± 0.48 * | 1.00 ± 0.40 | 1.72 ± 0.10 * |
| S-T90-6h | 1.09 ± 0.14 | 1.50 ± 0.23 | 5.01 ± 1.07 * | 4.62 ± 2.16 * | 1.89 ± 1.58 |
| S-T95-3h | 0.93 ± 0.24 | 1.52 ± 0.41 | 1.01 ± 0.78 | 2.92 ± 1.29 | 2.58 ± 1.29 |
| S-T95-6h | 0.78 ± 0.33 | 3.10 ± 2.09 | 2.39 ± 0.49 | 4.10 ± 2.04 * | 3.75 ± 1.21 * |
| H-T95-6h-B | 0.19 ± 0.06 | 0.26 ± 0.04 | 0.13 ± 0.03 | 0.19 ± 0.04 | 0.24 ± 0.04 |
| H-T95-6h-R | 0.44 ± 0.03 | 0.75 ± 0.08 | 0.69 ± 0.06 | 0.82 ± 0.09 | 0.81 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.B.; Lee, J.W.; Lee, D.E.; Na, S.C.; Jeong, D.E.; Hwang, D.I.; Kim, Y.S.; Park, C.B. Characteristics of Black Ginseng (Panax ginseng C.A. Mayer) Production Using Ginseng Stored at Low Temperature after Harvest. Metabolites 2021, 11, 98. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11020098
Oh HB, Lee JW, Lee DE, Na SC, Jeong DE, Hwang DI, Kim YS, Park CB. Characteristics of Black Ginseng (Panax ginseng C.A. Mayer) Production Using Ginseng Stored at Low Temperature after Harvest. Metabolites. 2021; 11(2):98. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11020098
Chicago/Turabian StyleOh, Hyo Bin, Ji Won Lee, Da Eun Lee, Soo Chang Na, Da Eun Jeong, Dae Il Hwang, Young Soo Kim, and Chung Berm Park. 2021. "Characteristics of Black Ginseng (Panax ginseng C.A. Mayer) Production Using Ginseng Stored at Low Temperature after Harvest" Metabolites 11, no. 2: 98. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11020098