Rietveld Analysis of Elpidite Framework Flexibility Using in Situ Powder XRD Data of Thermally Treated Samples
Abstract
:1. Introduction
1.1. Parameters Characterizing the Elpidite Framework Topology
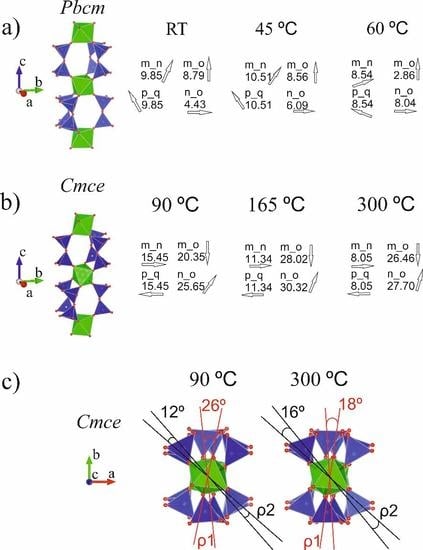
- due to symmetry conditions, two of the twist angles within a single Zr2Si6 CBU, designated hereinafter as ρ2, have equal values for all three space groups and, together with the third angle, ρ1, illustrate the zirconium octahedra mutual rotation within the CBU.
- S.G. Pma2—1 = ρ2 = 0; m_n = n_o = p_q ≠ 0; m_o = 0.
- S.G. Pbcm—ρ1 = ρ2 = 0; m_n = p_q ≠ m_o ≠ n_o; all tilt angles ≠ 0.
- S.G. Cmce—ρ1 ≠ ρ2 ≠ 0; m_n = p_q; all tilt angles ≠ 0; m_n azimuth = p_q azimuth + 180 °C.
1.2. Spatial Orientation of ZrO6 Octahedra in the Structures of Pma2-, Pbcm-, and Cmce-Elpidites
- The case of S.G. Pma2 is characterized by the identical spatial orientation of the ZrO6 polyhedra, which are located adjacently in four neighboring unit cells; therefore, they are all marked with 1 (Figure 2b).
- Although symmetrically related, the two zirconium octahedra (numbered 2 and 3, respectively) that fall within a single unit cell in the (001)-plane of the Pbcm-elpidite (KB) are not identically oriented due to the fact that in this case, the m_o tilt angle ≠ 0 (see previous section, Figure 1b). Translations along the a-direction repeats them in the same order within the neighboring unit cell (Figure 2c). This arrangement leads to a doubling of one of the ~7 Å parameters of the unit cell, as compared with the previously considered space group Pma2.
- At present, S.G. Cmce has only been registered for elpidite materials subjected to certain laboratory treatments (ion-exchange, heating and dehydration). The framework modifications that occur during the application of this procedure lead to a checkerboard arrangement of the Zr2Si6 structural units, subsequently causing the remaining unit cell parameter to be doubled to a value of approximately ~14 Å. Thus, two pairs of ZrO6, labelled 4 and 5, respectively, fall within a single unit cell (Figure 2d).
2. Materials and Methods
2.1. Analytical Procedure
2.2. General Notes on the Structure Rietveld Refinements
2.3. Choice of the Starting Crystal Structure Model
2.4. Notes on the Strategy Applied for the Structure Refinement Procedures in This Study
2.5. Visualization
- WinPLOTR utilities ver. June 2020 (Thierry Roisnel, Rennes, France) as a Windows tool [18] (powder X-ray diffraction patterns);
- VESTA ver. 3.3.2 (Koichi Momma, Tsukuba, Japan) [19] (visualization of certain structure and topological motives, calculation of torsion/dihedral angles, framework cation polyhedral volumes (PV); distortion indices (DI); and bond angle variances (BAV);
- Mercury ver. 3.7 (Cambridge Crystallographic Data Centre (CCDC), Cambridge, UK) [20] (tilt angle measurements).
3. Results
- approximately 70% (Pbcm) vs. 30% (Cmce) weight fractions for the 60 °C experiment;
- approximately 50% (Pbcm) vs. 50% (Cmce) weight fractions for the 75 °C experiment;
- approximately 24% (Pbcm) vs. 76% (Cmce) weight fractions for the 90 °C experiment.
- For the 60 °C experiment, only for the Pbcm-phase measurements provide evidence that the polyhedra bond length deviations do not exceed 5% of their ideal values and that the bond angle deviations reach values of less than 15% from their ideal values. The Cmce-phase undergoes considerable structure distortions in terms of bond angle values, and was subsequently excluded from consideration in the present study;
- For the 75 °C experiment, both phase structures were seriously affected by distortions, which makes their crystal chemistry implausible, and their results have not been reported further, here;
- For the 90 °C experiment, only the structure data for the Cmce-phase appear to be reliable, and those for the Pbcm-phase were defined as implausible and excluded from further consideration.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chukanov, N.V.; Pekov, I.V.; Rastsvetaeva, R.K. Crystal chemistry, properties and synthesis of microporous silicates containing transition elements. Russ. Chem. Rev. 2004, 73, 227–246. [Google Scholar] [CrossRef]
- Neronova, N.N.; Belov, N.V. Crystal structure of elpidite Na2ZrSi6O15.(H2O)3. Dimorphism of the dimetasilicate radical Si6O15. Dokl. Akad. Nauk SSSR 1963, 150, 642–645. [Google Scholar]
- Neronova, N.N.; Belov, N.V. Crystal structure of elpidite, Na2ZrSi6O15.(H2O)3. Sov. Phys. Crystallogr. 1964, 9, 700–705. [Google Scholar]
- Zubkova, N.V.; Nikolova, R.P.; Chukanov, N.V.; Kostov-Kytin, V.V.; Pekov, I.V.; Varlamov, D.A.; Larikova, T.S.; Kazheva, O.N.; Chervonnaya, N.A.; Shilov, G.V.; et al. Crystal Chemistry and Properties of Elpidite and Its Ag-Exchanged Forms. Minerals 2019, 9, 420. [Google Scholar] [CrossRef] [Green Version]
- Zubkova, N.V.; Ksenofontov, D.A.; Kabalov, Y.K.; Chukanov, N.V.; Nedel’ko, V.V. Dehydration-induced structural transformations of the microporous zirconosilicate elpidite. Inorg. Mater. 2011, 47, 506–512. [Google Scholar] [CrossRef]
- Sapozhnikov, A.N.; Kashaev, A.A. Features of the crystal structure of calcium-containing elpidite. Sov. Phys. Crystallogr. 1978, 23, 24–27. [Google Scholar]
- Grigor’eva, A.A.; Zubkova, N.V.; Pekov, I.V.; Kolitsch, U.; Pushcharovsky, D.Y.; Vigasina, M.F.; Giester, G.; Ðorðevic, T.; Tillmanns, E.; Chukanov, N.V. Crystal chemistry of elpidite from Khan Bogdo (Mongolia) and its K- and Rb-exchanged forms. Crystallogr. Rep. 2011, 56, 832–841. [Google Scholar] [CrossRef]
- Cannillo, E.; Rossi, G.; Ungaretti, L. The crystal structure of elpidite. Am. Mineral. J. Earth Planet. Mater. 1973, 58, 106–109. [Google Scholar]
- Cametti, G.; Armbruster, T.; Nagashima, M. Dehydration and thermal stability of elpidite: An in-situ single crystal X-ray diffraction study. Microporous Mesoporous Mater. 2016, 227, 81–87. [Google Scholar] [CrossRef]
- Nikolova, R.P.; Zubkova, N.Z.; Kostov-Kytin, V.V.; Chukanov, N.V.; Pekov, I.V. Elpidite framework flexibility exhibited upon ion-exchange and dehydration. In Proceedings of the Xth International Symposium “Mineral Diversity—Research and Preservation”, Earth and Man National Museum, Sofia, Bulgaria, 14–16 October 2019; pp. 175–183. [Google Scholar]
- Pyatenko, Y.A.; Voronkov, A.A. Comparative characteristics of the crystal-chemical functions of titanium and zirconium in mineral structures. Izv. Akad. Nauk SSSR Ser. Geol. 1977, 9, 77–88. [Google Scholar]
- Nikolova, R.; Fujiwara, K.; Nakayama, N.; Kostov-Kytin, V. Crystal structure of a new small–pore zirconosilicate Na2ZrSi2O7·H2O and its relation to stoichiometrically and topologically similar compounds. Solid State Sci. 2009, 11, 382–388. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS). Report LAUR 86-748; Los Alamos National Laboratory. Available online: https://11bm.xray.aps.anl.gov/documents/GSASManual.pdf (accessed on 10 May 2020).
- Toby, B.H. Expgui, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Petrova, R.; Nakayama, N.; Bakardjieva, S.; Bezdicka, P.; Kostov-Kytin, V. Temperature-induced phase transformations of the small-pore zirconosilicate Na2ZrSi2O7.H2O. Solid State Sci. 2011, 13, 1187–1190. [Google Scholar] [CrossRef]
- Kostov-Kytin, V.V.; Nikolova, R.P.; Lihareva, N.L. Two-stage protonation of a small-pore microporous zirconosilicate na Na2ZrSi2O7.H20. Bulg. Chem. Commun. 2012, 44, 83–90. [Google Scholar]
- EXPGUI—XRAY Subversion Server. Available online: https://subversion.xray.aps.anl.gov/EXPGUI/trunk/doc/expgui6R.html (accessed on 10 May 2020).
- Roisnel, T.; Rodriguez-Carvajal, J. Materials Science Forum. In Proceedings of the Seventh European Powder Diffraction Conference, Barcelona, Spain, 20–23 May 2000; Delhez, R., Mittenmeijer, E.J., Eds.; Scitec Publications, Ltd.: Baech, Switzerland, 2000; p. 118. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Streek, J.V.D. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Nedel’ko, V.V.; Chukanov, N.V.; Pekov, I.V. Dehydration kinetics of the microporous zirconosilicate elpidite. Inorg. Mater. 2011, 47, 502–505. [Google Scholar] [CrossRef]





| Material | In Situ Time-Resolved Powder X-ray Diffraction Studies (Selected Samples) | |||||
|---|---|---|---|---|---|---|
| Rietveld Statistics | Elpidite at 25 °C Single Phase Study | Elpidite at 45 °C Single Phase Study | Elpidite at 60 °C Two Phase Study | Elpidite at 90 °C Two Phase Study | Elpidite at 165 °C Single Phase Study | Elpidite at 300 °C Single Phase Study |
| Space Group, Z | Pbcm, 4 | Pbcm, 4 | Pbcm, 4 | Cmce, 8 | Cmce, 8 | Cmce, 8 |
| a (Å) | 7.0956(5) | 7.0961(5) | 7.092(1) | 14.089(1) | 14.083(1) | 14.126(1) |
| b (Å) | 14.6536(6) | 14.6525(6) | 14.6536(6) | 14.5385(9) | 14.4794(7) | 14.4907(8) |
| c (Å) | 14.5696(7) | 14.5711(7) | 14.5686(7) | 14.3241(9) | 14.3186(8) | 14.3309(8) |
| V (Å)3 | 1514.9(2) | 1515.00(2) | 1514.00(2) | 2934.0(4) | 2919.7(4) | 2933.4(4) |
| Rwp (%) | 12.46 | 12.81 | 10.10 | 9.33 | 11.68 | 11.88 |
| Rp (%) | 9.12 | 9.46 | 7.58 | 6.94 | 8.87 | 8.99 |
| Red-χ2 | 4.763 | 4.921 | 2.983 | 2.597 | 4.059 | 4.078 |
| Nobs | 1319 | 1322 | 2751 | 2641 | 1289 | 1294 |
| RF2 (%) | 10.12 | 10.44 | 7.38 | 6.00 | 7.58 | 7.73 |
| Nvar | 74 | 73 | 34 | 93 | 79 | 79 |
| No restraints | 18 | 18 | 36 | 36 | 18 | 18 |
| Total restraint χ2 contribution | 13.57 each | 6.44 each | 1.61 each | 5.15 each | 7.08 each | 2.04 each |
| Atom | x | y | z | Sof | Uiso, Å2 * |
|---|---|---|---|---|---|
| 0.4859(15) | 0.25 | 0.5 | 1 | 0.022(1) | |
| Zr | 0.496(4) | 0.25 | 0.5 | 1 | 0.02404 |
| 0.7766(28) | 0.3885(8) | 0.6474(9) | 1 | 0.021(1) | |
| Si1 | 0.783(5) | 0.388(1) | 0.647(1) | 1 | 0.02148 |
| 0.5046(30) | 0.0493(5) | 0.6466(7) | 1 | 0.021(1) | |
| Si2 | 0.509(5) | 0.0489(5) | 0.6492(8) | 1 | 0.02125 |
| 0.2147(29) | 0.3926(8) | 0.6472(9) | 1 | 0.021(1) | |
| Si3 | 0.222(5) | 0.391(1) | 0.645(2) | 1 | 0.02136 |
| 0.430(5) | 0.229(1) | 0.5000(0) | 1 | 0.044(3) | |
| Na1 | 0.418(8) | 0.2266(9) | 0.75 | 1 | 0.04135 |
| 0.012(3) | 0.5000(0) | 0.5000(0) | 1 | 0.012(3) | |
| Na2 | 0.014(10) | 0.25 | 0.5 | 1 | 0.00875 |
| 0.9961(31) | 0.4015(10) | 0.6361(13) | 1 | 0.036(2) | |
| O1 | 1.004(5) | 0.398(1) | 0.651(2) | 1 | 0.03043 |
| 0.727(8) | 0.3510(18) | 0.75 | 1 | 0.037(2) | |
| O2 | 0.720(11) | 0.354(3) | 0.75 | 1 | 0.03083 |
| 0.6738(32) | 0.3112(15) | 0.5894(15) | 1 | 0.036(2) | |
| O3 | 0.701(6) | 0.309(3) | 0.581(3) | 1 | 0.03033 |
| 0.659(4) | 0.4809(12) | 0.6195(19) | 1 | 0.038(2) | |
| O4 | 0.652(6) | 0.481(2) | 0.622(3) | 1 | 0.03203 |
| 0.448(7) | 0.0644(13) | 0.75 | 1 | 0.038(2) | |
| O5 | 0.447(9) | 0.070(1) | 0.75 | 1 | 0.03173 |
| 0.508(7) | 0.1390(8) | 0.5905(9) | 1 | 0.036(2) | |
| O6 | 0.528(9) | 0.1382(9) | 0.591(1) | 1 | 0.03023 |
| 0.310(4) | 0.4929(13) | 0.6274(20) | 1 | 0.035(2) | |
| O7 | 0.318(6) | 0.491(2) | 0.620(3) | 1 | 0.02923 |
| 0.290(7) | 0.3703(18) | 0.75 | 1 | 0.036(2) | |
| O8 | 0.298(10) | 0.367(3) | 0.75 | 1 | 0.03023 |
| 0.2711(32) | 0.3158(15) | 0.5745(16 | 1 | 0.036(2) | |
| O9 | 0.277(6) | 0.310(3) | 0.575(3) | 1 | 0.02993 |
| 0.023(8) | 0.113(1) | 0.581(1) | 1 | 0.055(6) | |
| O10 * | 0.054(8) | 0.1167(9) | 0.584(1) | 1 | 0.02058 |
| 0.157(8) | 0.200(2) | 0.75 | 1 | 0.085(6) | |
| O11 * | 0.120(10) | 0.196(2) | 0.75 | 1 | 0.05344 |
| Atom | x | y | z | Sof | Uiso, Å2 |
|---|---|---|---|---|---|
| 0.25 | 0.25 | 0.5 | 1 | 0.0115(7) | |
| Zr | 0.25 | 0.25 | 0.5 | 1 | 0.0187(9) |
| 0.2301(10) | 0.0505(5) | 0.6458(6) | 1 | 0.015(1) | |
| Si1 | 0.2318(10) | 0.0483(5) | 0.6444(6) | 1 | 0.016(1) |
| 0.3861(7) | 0.3789(5) | 0.6615(7) | 1 | 0.015(1) | |
| Si2 | 0.3951(9) | 0.3789(5) | 0.6633(5) | 1 | 0.016 (1) |
| 0.3875(6) | 0.0949(5) | 0.3727(7) | 1 | 0.015(1) | |
| Si3 | 0.3897(9) | 0.0959(5) | 0.3738(6) | 1 | 0.016(1) |
| 0.5 | 0.2435(19) | 0.516(1) | 1 | 0.057(3) | |
| Na1 | 0.5 | 0.2422(14) | 0.5174(13) | 1 | 0.069(4) |
| 0.25 | 0.2350(9) | 0.75 | 1 | 0.047(3) | |
| Na2 | 0.25 | 0.2405(10) | 0.75 | 1 | 0.058(4) |
| 0.5 | 0.0863(20) | 0.3813(22) | 1 | 0.022(2) | |
| O1 | 0.5 | 0.0633(18) | 0.3908(20) | 1 | 0.031(2) |
| 0.3608(17) | 0.3632(10) | 0.7723(7) | 1 | 0.020(2) | |
| O2 | 0.3603(18) | 0.3632(9) | 0.7727(8) | 1 | 0.033(2) |
| 0.1550(14) | 0.2003(11) | 0.4025(12) | 1 | 0.025(2) | |
| O3 | 0.1596(14) | 0.1953(11) | 0.3987(12) | 1 | 0.030(2) |
| 0.3256(13) | −0.0015(11) | 0.6130(15) | 1 | 0.024(2) | |
| O4 | 0.3250(13) | −0.0069(10) | 0.6159(14) | 1 | 0.033(2) |
| 0.25 | 0.0811(14) | 0.75 | 1 | 0.025(2) | |
| O5 | 0.25 | 0.0758(14) | 0.75 | 1 | 0.035(2) |
| 0.2188(18) | 0.1468(8) | 0.5964(10) | 1 | 0.021(2) | |
| O6 | 0.2186(19) | 0.1409(8) | 0.5882(10) | 1 | 0.031(2) |
| 0.1398(14) | 0.0164(8) | 0.3700(13) | 1 | 0.025(2) | |
| O7 | 0.1349(14) | 0.0138(8) | 0.3661(12) | 1 | 0.034(2) |
| 0 | 0.1205(16) | 0.3447(21) | 1 | 0.024(2) | |
| O8 | 0 | 0.1102(18) | 0.3778(17) | 1 | 0.033(2) |
| 0.3624(10) | 0.1764(9) | 0.4428(12) | 1 | 0.020(2) | |
| O9 | 0.3645(11) | 0.1755(10) | 0.4513(10) | 1 | 0.029(2) |
| 0.5 | 0.350(2) | 0.411(2) | 1 | 0.079(11) | |
| Ow1 * | 0.5 | 0.341(2) | 0.404(2) | 0.79(3) | 0.063 |
| 0.5 | 0.135(2) | 0.691(2) | 1 | 0.129(15) | |
| Ow11 * | 0.5 | 0.165(3) | 0.368(4) | 0.73(2) | 0.129 |
| In Situ Time-Resolved Powder X-ray Diffraction Studies (Selected Samples, RWf = 20,000) | |||||
|---|---|---|---|---|---|
| Atomic Pair | (Pbcm) | (Pbcm) | Atomic Pair | (Cmce) | (Cmce) |
| at 25 °C | at 60 °C | at 90 °C | at 165 °C | ||
| Zr-O3[x2] | 2.071(10) | 2.0502(2) | Zr-O3[x2] | 2.064(7) | 2.086(9) |
| Zr-O6[x2] | 2.099(8) | 2.11419(7) | Zr-O6[x2] | 2.086(7) | 2.071(8) |
| Zr-O9[x2] | 2.103(10) | 2.0988(2) | Zr-O9[x2] | 2.079(7) | 2.061(9) |
| Si1-O1 | 1.578(13) | 1.5712(2) | Si1-O7 | 1.617(10) | 1.641(13) |
| Si1-O2 | 1.632(12) | 1.64881(7) | Si1-O5 | 1.583(8) | 1.585(8) |
| Si1-O3 | 1.590(11) | 1.62584(5) | Si1-O6 | 1.577(9) | 1.574(10) |
| Si1-O4 | 1.640(13) | 1.68435(9) | Si1-O4 | 1.614(10) | 1.589(12) |
| Si2-O4 | 1.585(13) | 1.5660(1) | Si2-O3 | 1.581(9) | 1.592(10) |
| Si2-O5 | 1.575(12) | 1.56296(7) | Si2-O7 | 1.630(9) | 1.664(11) |
| Si2-O6 | 1.549(9) | 1.57068(5) | Si2-O8 | 1.607(9) | 1.597(10) |
| Si2-O7 | 1.579(14) | 1.5477(1) | Si2-O2 | 1.643(10) | 1.657(11) |
| Si3-O1 | 1.565(13) | 1.5535(2) | Si3-O4 | 1.627(9) | 1.585(11) |
| Si3-O7 | 1.643(12) | 1.65868(7) | Si3-O2 | 1.606(10) | 1.619(12) |
| Si3-O8 | 1.623(13) | 1.66139(7) | Si3-O9 | 1.594(9) | 1.639(10) |
| Si3-O9 | 1.596(12) | 1.61252(5) | Si3-O1 | 1.594(9) | 1.642(10) |
| N | Twist Angle ρ1 (°) | Twist Angle ρ2 (°) | Tilt Angle m_n/Azimuth (°) | Tilt Angle m_o/Azimuth (°) | Tilt Angle n_o/Azimuth (°) | Tilt Angle p_q/Azimuth (°) | PV, ZrO6, (Å)3 | DI Zr-O, (Å) | BAV, ZrO6, (°) | Averaged PV, SiO4, (Å)3 | Averaged DI Si-O, (Å) | Averaged BAV, SiO4 (°)2 | Compound, Locality, Exper. Conditions [ref] Results of Quant. Analysis Space Group Unit Cell Parameters: a, b, c, (Å), V (Å)3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Single crystal studies | |||||||||||||
| 1 | 0 | 0 | 5.77/95 | 0 | 5.77/265 | 5.77/265 | 11.85 | 0.004 | 1.9011 | 2.1374 | 0.01767 | 8.5015 | Elpidite init., Lovozero (L), RT, Na2ZrSi6O15·3H2O (CCDC with CSD deposition number 1,937,293 [4]); Pma2 14.6127(7); 7.3383(4), 7.1148(3); 762.94(6) |
| 2 | 0 | 0 | 6.07/65 | 2.65/0 | 5.46/90 | 6.07/295 | 12.00 | 0.006 | 3.079 | 2.1541 | 0.01106 | 5.70237 | Elpidite init., Khan Bogdo (KB), RT, [7]; Pbcm 7.131(12, 14.685(1), 14.6349(15); 1532.6(3) |
| 3 | 0 | 0 | 6.53/60 | 3.19/0 | 5.69/90 | 6.53/300 | 11.87 | 0.005 | 3.404 | 2.1490 | 0.0107 | 6.590 | Elpidite init., Mont Saint-Hilaire (MSH), RT, Na2ZrSi6O15·3H2O [9]; Pbcm 7.1134(1), 14.6796(2), 14.6030(2); 1524.87(4) |
| 4 | 17.62 | 9.17 | 8.17/90 | 31.40/180 | 32.50/15 | 8.17/270 | 11.93 | 0.004 | 1.362 | 2.1453 | 0.0086 | 7.619 | (MSH) elpidite, in situ heated at 100 °C and partially dehydrated, Na2ZrSi6O15·1.8H2O [9]; Cmce (64) 14.1260(5), 14.5734(5), 14.3627(5); 2956.8(2) |
| 5 | 18.62 | 9.19 | 6.41/90 | 30.04/180 | 30.74/15 | 6.41/270 | 11.87 | 0.009 | 2.839 | 2.1333 | 0.00863 | 7.4124 | (MSH) elpidite, in situ heated at 250 °C anhydrous, Na2ZrSi6O15 [9]; Cmce 14.1271(4), 14.5110(4), 14.3533(4); 2942.4(1) |
| PXRD studies, this work | |||||||||||||
| 6 | 0 | 0 | 9.85/25 | 8.79/0 | 4.43/90 | 9.85/330 | 12.07 | 0.006 | 24.25 | 2.0502 | 0.01371 | 49.11307 | Na2ZrSi6O15·3H2O, (L), RT, single phase study; Pbcm 7.0956(5), 14.6536(6); 14.5696(7); 1514.9(2) |
| 7 | 0 | 0 | 10.51/35 | 8.56/0 | 6.09/90 | 10.51/325 | 12.00 | 0.011 | 25.66 | 2.0650 | 0.01758 | 53.58603 | Na2ZrSi6O15·3H2O, (L), 45 °C, single phase study; Pbcm 7.0961(5), 14.6525(6), 14.5711(7); 1515.04(16) |
| 8 | 0 | 0 | 8.54/70 | 2.86/0 | 8.04/90 | 8.54/290 | 12.03 | 0.012 | 20.33 | 2.0787 | 0.01637 | 59.06697 | Na2ZrSi6O15·3H2O, (L), 60 °C, two phase study; quant. analysis Pbcm:Cmce = 70:30; Pbcm 7.0920(10), 14.6536(6), 14.5686(7); 1514.0(2) |
| 9 | 25.68 | 12.14 | 15.45/90 | 20.35/180 | 25.65/35 | 15.45/270 | 11.89 | 0.004 | 9.998 | 2.10093 | 0.01037 | 29.88363 | Na2ZrSi6O15·2H2O, (L), 90 °C, two phase study; quant. analysis Pbcm:Cmce = 30:70; Cmce 14.0886(13), 14.5385(9), 14.3241(9); 2934.0(4) |
| 10 | 23.15 | 14.86 | 11.34/90 | 28.02/180 | 30.32/20 | 11.34/270 | 11.86 | 0.004 | 3.078 | 2.11247 | 0.01522 | 60.31813 | Na2ZrSi6O15·1.5H2O, (L), 165 °C, single phase study; Cmce 14.0829(12), 14.4794(7), 14.3186(8); 2919.7(4) |
| 11 | 17.70 | 15.46 | 8.05/90 | 26.46/180 | 27.70/17 | 8.05/270 | 11.58 | 0.007 | 2.729 | 2.103 | 0.02232 | 47.4003 | Na2ZrSi6O15·H2O, (L), 300 °C, single phase study; Cmce 14.1255(13), 14.4907(8), 14.3309(8); 2933.4(4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostov-Kytin, V.V.; Kerestedjian, T.N. Rietveld Analysis of Elpidite Framework Flexibility Using in Situ Powder XRD Data of Thermally Treated Samples. Minerals 2020, 10, 639. https://0-doi-org.brum.beds.ac.uk/10.3390/min10070639
Kostov-Kytin VV, Kerestedjian TN. Rietveld Analysis of Elpidite Framework Flexibility Using in Situ Powder XRD Data of Thermally Treated Samples. Minerals. 2020; 10(7):639. https://0-doi-org.brum.beds.ac.uk/10.3390/min10070639
Chicago/Turabian StyleKostov-Kytin, Vladislav V., and Thomas N. Kerestedjian. 2020. "Rietveld Analysis of Elpidite Framework Flexibility Using in Situ Powder XRD Data of Thermally Treated Samples" Minerals 10, no. 7: 639. https://0-doi-org.brum.beds.ac.uk/10.3390/min10070639