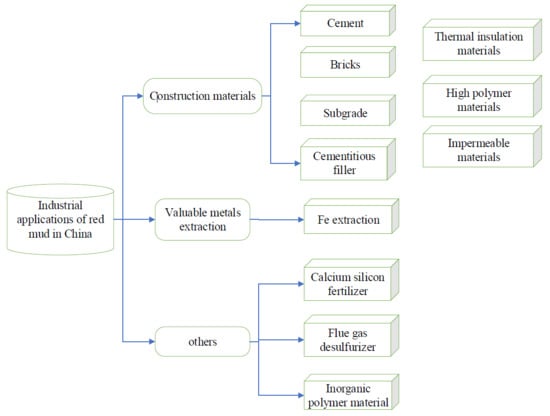
3.1. Cement
Ordinary Portland cement is generally sintered from a mixture of limestone, quartz, iron ore, clay or bauxite [
42]. RM has some cementitious activity, and its compositions are similar with Portland cement, which means that RM can be used to produce cement. However, its hydraulic activity is low [
55], so RM is usually synergistic with gypsum, blast furnace slag, fly ash, coal gangue, lime and other substances to stimulate its cementitious activity.
Shandong Aluminum Company, as China’s first alumina plant, began to use RM very early. As early as 1958, the company successfully produced Portland cement using sintering RM and limestone tailings as the main raw materials, adopting a wet production process, and 5% gypsum and no more than 15% active mixed materials were also added [
62]. The producing process of RM-based cement was similar with Portland cement, which is shown in
Figure 4. The strength of RM-based cement reached 48–52 MPa, fully meeting the requirements of ordinary 425R cement. However, due to the high alkali content of RM (2.0–10.0% Na
2O) [
63], the addition ratio of RM was restricted and only about 25% [
64].
In order to increase the addition ratio of RM in raw material without removing alkali, the company carried out many industrial experiments of adding mineralization agent to cement. The results showed that adding 0.5% fluorite mineralizing agent (containing 40–60% CaF
2) to raw materials can promote the normal absorption of free calcium oxide in the sintering process. The clinker could be sintered normally with high alkali content (up to 1.8%), and the proportion of RM can be increased to 40–45% without scarifying the mechanical properties [
65]. This technology was successfully applied to production in the early 1980s.
Since 1954, Shandong Aluminum Company has been producing RM-based Portland cement with an annual output of 1.6 million tons of clinker. By 1997, the company had produced more than 20 million tons of cement and comprehensively utilized more than 6 million tons of RM, which is by far the largest RM comprehensive utilization method. The annual average ratio of RM was 20–38%, the utilization amount of RM was 200–420 kg/t, and the comprehensive utilization rate of RM was 30–55% [
66].
However, the wet process was to grind raw materials into slurry (water content was 32–40%) for calcination, requiring a lot of energy to dry raw material and making its cost high [
67]. Dry process is to grind raw materials into dry powder for calcination with low energy consumption. However, it is difficult for the dry process to make raw materials fully mix, limiting the application of the dry process. With the technological progress in the cement industry, it is possible to replace the wet process with the dry process. Moreover, Shandong Aluminum Company solved the problem of RM alkali removal. They used lime as main dealkalization agent to carry out dealkalization at a low temperature of 70–90 °C, a calcium/sodium ratio of about 3.0 and a liquid/solid ratio of 3.0. The dealkalization rate of RM reached more than 80%, and RM alkali was successfully reduced from 2.5–3.5% to less than 1.0%, satisfying the requirement of low alkali cement [
68]. In 2003, the company’s first 3000 t/d new dry cement clinker production line was put into operation using sintering RM and combined RM as raw material. The addition ratio of RM was up to 45%, and the quality of cement was improved from number 425 Portland cement to number 525 Portland cement [
69]. Drawing on a similar process, Guangxi Aluminum Company started to build a new dry cement production line of 3200 t/d clinker and 6600 t/d clinker in 2015. It was estimated that the company would discharge 1.02 million tons of RM, limestone waste residue, fly ash, slag, desulfurization gypsum and other industrial waste residue every year after the completion of the project.
Apart producing Portland cement, RM was also used to produce other cement. Taking advantage of the strong resistance to sulfate erosion, Shandong Aluminum Company developed sulfate-resistant RM cement and the consumption of RM of cement reached 600–800 kg/t. The products were mainly used for salinization projects with seawater contact, anti-corrosion and underwater engineering of the salinization industry. The addition ratio of RM was up to 60%, and the output reached 2000–4000 t/year [
65]. On the basis of using RM to produce Portland cement, the plant adjusted the grinding size of cement powder and properly controlling the content of tricalcium aluminate in clinker, and successfully produced oil well cement. The production capacity reached 100,000–200,000 t/year and produced oil well cement was mainly used in the SINOPEC Shengli oilfield [
65]. Entering the 1990s, the oil well cement of Shandong aluminum plant was suspended due to the Shengli oilfield building its own cement factory [
65].
Long–time industrial practices showed that RM can be used to produce Portland cement and other cements. Produced RM Portland cement also showed comparable mechanical properties compared with ordinary Portland cement. Moreover, RM Portland cement has some better characteristics, such as high early strength, moderate setting time, good freezing–thawing resisting performance, good sulfate resistance and erosion resistance. It is suitable for components requiring high construction speed and high early strength and a working environment requiring high sulfate content.
The high alkalinity of RM is the main problem limiting its utilization in the cement industry (even building industry). The alkali in RM mainly exists in the state of soluble alkali and chemical binding alkali. Soluble alkali is an important component of RM alkalinity, among which soluble alkali accounts for about 40–60% of the total alkali amount. The soluble alkali mainly exists in the forms of NaOH, Na
2CO
3, NaHCO
3, NaAl(OH)
4, Na
2SiO
3, KOH and K
2CO
3 [
70]. Additionally, it is easily soluble in the liquid phase, leading to the increase in pH value. The chemical binding alkali is the sodium silicon slag, which was formed by the reaction of SiO
2 in bauxite with sodium aluminate solution. Additionally, chemical binding alkali mainly existed in the forms of cancrinite, sodalite, zeolite and amorphous sodium aluminosilicate [
71]. The cement industry is strict with the alkali content of raw materials, which is generally required to be less than 1%. It was showed that the addition ratio of RM was mainly depended on the amount of alkali substances existed in RM (only about 20–30%). Although some feasible de-alkali method was putted, the high alkalinity of RM is still the main problem limiting the use of RM in the cement industry (even whole building industry). Moreover, it could be found that the market acceptance of RM products is low from the suspension of oil well cement. So, in addition to making RM cement have a higher performance, it was also necessary for government to increase the publicity of RM products and encourage the use of RM products.
3.2. Sintered Bricks
Traditionally, sintered bricks were made from clay, shale, coal gangue and other substances fired at a high temperature [
72]. The production process of sintered RM brick was similar to the traditional sintered brick, which were normally produced using RM, shale and other materials as main materials after comminuting, mixing, shaping, drying and roasting. RM has been successfully used to produce various bricks such as insulation brick, permeable brick, landscape brick and pavement brick.
The compositions of RM are relatively stable and can be regarded as an inert component when temperature is less than 900 °C [
30], which makes the use of RM as raw material to produce thermal insulation and refractory insulated material possible. Long time studies showed that RM brick has good properties and its radioactivity was in an acceptable range (internal exposure index (I
Ra) and external exposure index (I
r) was less than 1.0 according to GB 6566-2010 (Limits of radionuclides in building materials)) [
2].
In 2009, Shanxi aluminum plant successfully developed refractory thermal insulation bricks for industrial kilns by using RM and fly ash as raw material, and the addition ratio of RM and fly ash was more than 50%. A production line of RM fly ash refractory thermal insulation bricks with a production capacity of 12,000 t/year was built in 2010 [
73]. Up to now, the company has formed a production line of RM fly ash refractory insulation bricks with an annual output of 100,000 t, and the addition ratio of RM accounted for about 30% of the quality [
73]. The annual utilization amount of RM reached about 30,000 t, and the RM fly ash firebrick showed comparable mechanical properties compared with traditional sintered brick. Moreover, it has many advantages, such as high porosity, low density and low thermal conductivity.
For expanding the application range of sintered RM bricks, Guizhou Building Materials Science Research and Design Institute successfully produced sintered RM pavement brick using RM, shale and coal gangue as the main raw materials, among which the mixing amount of RM was up to 40–50%, and the mechanical properties satisfied the requirement of sintered pavement brick (GB/T 26001-2010). The average compressive strength was more than 25 MPa and frost would not occur. Moreover, the radioactive elements were satisfied with the requirement of limits of radionuclides in building materials (GB 6566-2010). At present, an industrial production line, with an annual output of 40 million RM sintered pavement bricks, has been built. Apart from using sintering RM to produce sintered bricks, a company in China’s Shandong province also successfully made use of Bayer RM to produce landscape bricks in 2018 by adding a small amount of ceramic raw materials, among which the addition amount of RM was more than 50%. The average compressive strength reached about 80 MPa, and the resistance of acid and alkaline was over 90%. The internal exposure index (IRa) and external index (Ir) were 0.2 and 0.9, respectively, both of which met the requirement according to the limits of radionuclides in building materials (GB 6566-2010) (Ira and Ir was required to be less than 1.0). At present, the company is docking with the local aluminum plant for the use of this achievement.
RM permeable brick belongs to another patent technology of RM sintered brick [
74]. The technology solves the problem of the dissolution of alkali metals and heavy metals in RM, so that RM can be safely used as raw material in permeable pavement materials, and the comprehensive utilization rate of RM waste residue can reach 50–70%. In 2018, a company in Shandong province built a fully automatic production line of ecological RM permeable brick with a daily output of 3000 square meters, which can digest nearly 100,000 t of RM every year, and the addition ratio of RM reached 80%. The thickness of RM permeable brick is 3.5 cm, and the bending strength of RM brick reached 6–8 MPa when the national standard is 3.2 MPa. Radioactivity, heavy metal dissolution and other indicators met the requirements of building materials according to GB 6566-2010. Moreover, RM brick has excellent performance in terms of water permeability, anti-freezing and thawing, and anti-sliding property [
42]. Economically, the price of RM brick was the same as that of clay brick. Until now, the brick has been widely used in pavement laying (
Figure 5), greatly improving pavement permeability, anti-sliding and noise reduction.
3.3. Non-Fired Bricks
In the calcination process of sintered bricks, a large number of environmental pollutants were released, large amounts of energy consumption were consumed, and many greenhouse gases were produced [
19]. Nowadays, the production of sintered bricks has been restricted, even have forbidden producing clay bricks in global some areas, which made the production of non-fired bricks become the development direction in the brick industry. RM contains a large amount of dicalcium silicate (C
2S), some tricalcium silicate (C
3S) and tricalcium aluminate (C
3A) phase, which makes RM have some potential cementitious activity and water hardness [
74]. The active index of RM is more than 90%, and it can be used to produce non-fired bricks by adding some activators, curing agents and aggregates. Gel materials are generated through a series of hydration reactions and make the combination between different particles closer, so as to meet relevant national standard of baking-free brick [
75].
Shandong Aluminum Company successfully produced RM non-fired brick by natural curing and autoclave curing using sintering RM, fly ash and mining slag as the main materials. The raw materials of the two processes have been shown in
Table 4. The 28-day compressive and flexural strength of non-fired brick by natural curing and autoclave curing reached 17.5 and 2.8 MPa, 15.6 and 4.8 MPa, respectively, meeting the requirement of MU15 brick according to relevant Chinese standards (GB 11945-1999). Both types of non-fired brick are already in production. Economically, the production cost with natural curing could be controlled below 0.11 Yuan/block, and the production cost with autoclave curing could be controlled below 0.14 Yuan/block, which showed better economy [
76].
The company also successfully developed a non-steaming and non-firing brick based on RM:fly ash:aggregate of 30%:23%–30%:30%. The 28-day compressive strength was more than 9 MPa, which was not satisfied with the requirement of MU15 bricks [
78]. Then they took 50% fly ash, 10% calcium carbide slag, 10% sodium silicate slag, 20% RM, 10% stone debris and 2% desulfurization gypsum as raw materials, and successfully made RM brick using the semi-dry pressing molding process. The 28-day compressive and bending strength was up to 16.6 MPa and 4.2 MPa [
79], meeting requirement of MU15 brick. In cooperation with Huazhong University of Science and Technology, the company took RM and fly ash as the main raw materials, and prepared RM fly ash brick adopting natural curing. Moreover, some lime, gypsum and 32.5 ordinary Portland cement were added as curing agent and activator. The ratio of RM:fly ash:aggregate:gypsum:lime:cement are 33%:24%:30%:2%:10%:1%. The bending strength and compressive strength reached 3.22 and 22.42 MPa, meeting the requirement of MU15 brick according to GB 11945-1999. The brick has been already in production, 18 million of RM fly ash bricks were produced every year and more than 20,000 t of RM was consumed annually [
80].
Many actual practices showed that RM can be used to produce sintered bricks and non-sintered bricks. Additionally, taking advantage of the high porosity of RM, RM bricks are more suitable for porous bricks. Although there are some feasible RM utilization methods in the brick industry, there still are some key problems that need to be solved. Based on the above, it could be found that the RM addition ratio was low (only about 20–30%), and it was hard to take advantage of the high waste consumption of brick. Moreover, the high alkalinity of RM still poses a big problem for its utilization in building materials, which easily caused efflorescence. Additionally, the high cost of activator and the radioactivity of RM brick still limited its utilization. How to coordinate the use of RM and other industrial wastes to the greatest extent to make bricks and realize the complete control of waste by waste may be the future development trend.
3.4. Subgrade
Not only can the application of the piled RM to the roadbed filling achieve the large-scale utilization of RM, but also it can save the natural resources [
8]. However, some properties of RM, such as its high fineness, strong water holding capacity and poor water stability, make the engineering properties of RM cannot meet the technical requirements of subgrade filling. RM is needed to be improved mainly by chemical method. The chemically improved method is normally to change the microstructure of soil by adding lime, cement, fly ash and other inorganic cementitious materials, which is to react physically and chemically with soil particles and then improve the strength, stiffness and water stability of RM subgrade [
43].
Shandong Aluminum Company built a 4-km-long RM roadbed demonstration section in 2015 (as seen in
Figure 6), which used sintering RM, fly ash and lime as the main raw materials and the ratio was 75%:15%:10% (dry mass ratio). The 7 and 28 day average compressive strength reached 1.2 and 3 MPa, which met the strength requirements of grade-I lime-stabilized soil and highway [
81]. This was the first sintering RM pavement base project applied in the actual highway in China, which consumed more than 20,000 t of sintering RM. It was one of the projects with the largest utilization amounts of RM in recent years and has been in normal use until now [
82]. In 2008, the company provided the same formula and cooperated with local municipal highway bureau to build a road with 500 m long and 27 m wide, consuming nearly 4000 t of sintering RM. Actual practices showed that the whole test section was in good operation and basically qualified with national standards. [
83]. The 7 and 28 day compressive strength reached 1.96 and 2.6 MPa, which met the strength requirements of the first grade of the stable soil layer of lime and the expressway. Economically, the construction cost of RM base was 10–20 Yuan cheaper than several common base materials, such as limestone soil and lime-ash gravel, under the same transportation distance [
84].
Cooperating with Beijing Mining and Metallurgy Research Institute, Pingguo Aluminum Company has also developed the first RM basic road and a new RM concrete pavement in China through the comprehensive solidificaion technologies, including alkali stabilization, ion exchange, RM activation and pressure molding. The industrial test of 800 m RM basic road, 500 m RM concrete pavement and 5 km expansion industrial test have been completed. The main raw materials of basic road were RM:lime:fly ash with the ratio of 80%:10%:10% and the curing agent were provided by Beijing General Research Institute of Mining Industry. The density was 1.84 g/cm
3, and the 7-day and 28-day compressive strength reached 3.55 MPa and 4.25 MPa. The ratio of concrete pavement was RM:fly ash:cement:graded gravel:curing agent = 30–35%:5–10%:10–15%:40–50%:0.2–0.25% [
85]. RM was consumed at 2.04 t/m for the basic road and 0.63 t/m for the concrete pavement, with a total RM consumption of 1947 t [
86]. After nearly a year test like sun exposure, rain erosion and large-tonnage vehicles unbalanced driving, the operation was excellent, meeting the requirements of high-grade highway. In recent years, Pingguo Aluminum Company has been promoting this achievement, and has been laying 22 km RM roads and totally consuming 117,500 t of RM [
86].
A company in China’s Shandong province has realized the world’s first application of Bayer RM in the construction of actual highway engineering, and successfully applied it to the expressway. In 2018, 25,000 t of RM was used on the subgrade of the expressway and the construction diagram of RM pavement has shown in
Figure 7. The RM based concrete was built through mixing and compacting, which was based on RM and RM modifier in a ratio of 92:8. RM modifier was made based on phosphorus gypsum and cement as main material, also some heavy metal reducing agent, complexing agent and curing modification materials were added. The RM modifier had a good solidification and stability effect on the pollutants in the original RM, and effectively reduced the dissolution of pollutants, making RM concrete meet the requirements of environmental protection. The 7 d compressive strength reached 3.1 MPa, fully meeting the requirement of the highway. The subgrade has been successfully applied to the highway, and the company’s achievements have been successfully used in many RM highway projects, expansion projects and the national highway G309 construction project in China. Modified RM could be used to replace ordinary lime soil and cement, and practical utilization showed that the price was 10–30% cheaper [
87].
The University of Science and Technology, Beijing, successfully produced a new pavement material using RM, coal gangue, fly ash, desulfurization gypsum, furnace slag and a small amount of cement. The addition ratio of solid wastes is as high as 97%, and the mechanical properties were good. The 7-day compressive strength was up to 6.2 MPa, the leaching of sodium element in 7-day was 0.0013% (mass percentage), and the construction process was convenient. This achievement has been successfully applied in the road project of Huaxing Aluminum Company in China’s Shanxi province, and actual practices showed that its application effect was good.
With the characteristics of small density, fine particles and good hydration activity of sintering RM, Shandong Transportation Research Institute prepared high fluidity and light-weight cementitious pouring materials combining sintering RM with some cement, fly ash and water-reducing agent. The ratio of sintering RM:cement:fly ash (mass ratio) was 84:8:8 and the content of water-reducing agent was 1% of the quality of dry powder. The engineering was successfully applied in backfilling on highway expansion project. Long-time actual properties tests showed that the strength and working performance of the pouring entity were good, which has high practicability and application value [
88].
A road base course of 50 m in length, 15 m in width and 24 cm in thickness was laid by Henan Institute of Traffic Science with lime:fly ash:RM as 6:24:70. The construction practice and application process showed that lime fly-ash-stabilized RM base had good dynamic stability, dry shrinkage and warm shrinkage [
89]. They also used lime:RM:soil as 6:36:58 to lay two lime RM stabilized soil bases with a length of 150 m, a width of 15 m, a thickness of 24 cm and a length of 2000 m and a width of 7 m. A total of 14,000 m
2 of RM was consumed [
89]. Two years of testing showed that the RM base has the advantages of high strength, good plate property, water stability and frost resistance. Actual practice showed that the cost of lime RM mixture base was about 30% lower than that of lime clay base due to the test section being close to the RM producing area. Moreover, an appropriate amount of RM was also applied to acid soil to improve the acid soil by virtue of the strong alkalinity of RM. This method has been applied in some acid mines [
66].
Although some properties make RM cannot meet the requirement of subgrade, but many actual practices of RM in subgrade showed that it can be immensely activated by inorganic cementitious activators such as lime, fly ash, coal gangue and slag. These activators can react physically and chemically with RM, changing its microstructure and improving its strength, stiffness and water stability. At present, RM can be well solidified and would not pollute the environment. However, in the longer run, it still need a lot of time to confirm. Also it was showed that there are some problems limited the use of RM in subgrade such as the cost and stimulating effect of RM activators, the cost and effect of impermeable material and the solidified ability of RM modifiers.
3.5. Thermal Insulation Material
Micro-porous calcium silicate thermal insulation material is a new type of environmental protection and energy saving material. It has some advantages, such as low bulk density and thermal conductivity, high compressive and flexural strength and reusability, and is generally used at high temperature parts of 650–1000 °C [
90]. It is normally produced by a dynamic method using materials rich in active silica as main materials and lime, fiber as reinforcement materials [
91].
Using RM to replace diatomite and combining it with lime, bentonite and admixture, Shandong aluminum plant successfully developed RM micro-porous calcium silicate insulation material. The addition ratio of RM reached 30%, and the production process and physical properties of the product are shown in
Figure 8 and
Table 5. Its mechanical properties were excellent (the density was 215 kg/cm
3, the flexural and compressive strength reached 0.31 and 0.91 MPa, the thermal conductivity was 0.0554 W/(m·K), and the maxing affordable temperature was up to 650 °C), fully meeting the relevant national standards [
91]. The product was put into industrial production in 2001, and the production line of calcium silicate insulation material with an annual output of 12,000 m
3 was built. Practical practices showed that the production line process stability, product quality and economic benefits were good [
91].
Inorganic fiber is another kind of thermal insulation material made from minerals, and it is generally produced by the ore and coke in suitable proportion through high temperature melting and centrifugation. It is usually sprayed onto the surface of objects as an insulating material. Acidity coefficient (MK) and viscosity coefficient (MB) are commonly used to represent and control its performance [
43]. A high MK value has a good thermal insulation effect, and is generally 1.4–1.6 and 1.1–1.4 for rock wool and mineral wool. The larger the MB value, the more viscous the melt, and the fiber is not easy to slim. The MB value is generally between 1.0 and 2.0 [
92].
where
m is the number of holes.
The chemical compositions of inorganic fiber belong to SiO
2-A1
2O
3-CaO-MgO system, and RM contains oxides required by inorganic fiber, which could be used to produce inorganic fiber by adding fly ash, carbide slag, blast furnace slag and other solid wastes [
92]. A company in Henan province has successfully produced qualified inorganic fiber by mixing RM, fly ash and calcium carbide slag on an industrial scale, and the ratio of RM, fly ash and carbide slag was 35–40%:23–28%:32–37%. The flow chart of the industrial preparation method and the chemical composition of RM inorganic fiber were shown in
Figure 9 and
Table 6. The thermal resistance of fiberboard with a density of 70 kg/m
3 was 2.21 (m
2∙K∙w
−1), and the fiber formation rate reached about 80% [
92].
A company in Shandong province also successfully produced inorganic fiber using RM as the main material. The RM is mixed with curing agent and acidity coefficient regulator and then fused with coke at a high temperature, and inorganic fiber was obtained after centrifugation. The addition ratio of RM reached more than 70%. Additionally, the pollution of RM was effectively controlled, and the inorganic fiber produced reached the level of rock wool. Economically, the production process was simple and cost low. In 2014, another company in Shandong province successfully produced qualified rock wool with RM as the main raw material and realized industrial production. By adding some additives, the acid coefficient (SiO
2, Al
2O
3):(CaO, MgO) was greater than 1.6, and CaO:MgO was greater than 1. The rock wool has good water resistance, which overcomes the defect of declining stability of slag cotton fiber in wet environment [
93].
The high fineness and high BET made RM could be used as thermal insulation material. However, its compositions could not better satisfy with insulation material. It was necessary to add some substances to adjust chemical compositions, which leads to the reduction of RM content. For controlling heavy metal and radioactive elements, some expensive modifier may be needed. It may increase the cost of RM products and make the use of RM uneconomic, making it hard to use on an industrial scale. So, there are still many areas for improvement, such as developing cheaper and/or effective modifier.
3.6. High Polymer Material
RM plastic is a new energy-saving and environmentally friendly building material, which provides a new way for the utilization of RM. It was produced based on PVC resin (or waste PVC plastic) as a basis material and pretreated RM as a filling agent after kneading, banburying, calendering or blow molding. Moreover, some waste engine oil and phthalate esters were added as processing aids and plasticizer, and sometimes some glass fiber, plant fiber and synthetic fiber was added as reinforcing agent [
72]. RM plastic normally consisted of 20–80% recycled PVC, 5–80% RM, 0–20% waste oil, 0–50% DOP and 0–20% other fillers [
94].
Compared with ordinary PVC plastics, it has higher tensile strength and elastic retention force, higher wear, corrosion, acid and alkali resistance, self-extinguishing ability, better light shielding and aging resistance. Economically, its cost is low, which is 10–20% cheaper compared with general PVC plastic [
95]. The mechanism is as follows: (1) CaO, SiO
2 and TiO
2 in RM are high quality fillers for PVC; (2) RM contains a large number of free alkali and CaO, and can quickly absorb the HCl released from PVC aging and delay the chain reaction. The life of RM plastic is 2 to 3 times longer than that of ordinary PVC products; (3) Fe
2O
3 and TiO
2 in RM are good light shielding agents, which can absorb ultraviolet rays and delay photoaging; (4) RM is combined with waste PVC to form a mesh body to strengthen the plastic, which is a filler with a reinforcing effect on PVC [
94]; (5) the fluidity of RM is better than other fillers, so that the plastic has good processing performance [
68].
RM plastic has been made into hard and soft products and is widely used in industry, agriculture and daily life (some was shown in
Figure 10). Soft RM plastic can be used as storage bags for grain, fruits and vegetables feed [
96], and solar water heater [
97]. The hard products can be used as corrugated board [
98], floor tiles [
95], plastic round pipes [
95], biogas fermentation tanks and gas storage tanks [
99], artificial leather [
100], and pipes [
101].
3.7. Cementitious Filler
RM contains a large number of hydraulic cementitious minerals, such as dicalcium silicate and tetralcium ferroaluminate, which makes RM have a strong potential activity. However, the particles of dicalcium silicate are covered by the generated calcium silicate hydrate (C–S–H), which greatly reduces the reaction speed of dicalcium silicate. The infiltration and diffusion of reaction molecules are very slow, so that the RM becomes inert, and it only has weak or no hydraulic activity and it cannot be solidified by itself. Some activators such as limestone and gypsum have a significant excitation effect on RM, making RM generate a large number of hydraulic silicates and aluminate gel, and generated sufficient strength [
19,
102,
103].
Shandong Aluminum Industry produced cementitious filling material using sintering RM, fly ash and lime as main materials, in which lime was used as RM activator and the ratio of water:RM:fly ash:lime was 2.43:2:1:0.57. The achievement was successfully applied to the mine filling of its own bauxite mine in the 1990s. Actual practices showed that its application effect is good, and the RM filling material has good working behavior and good fluidity [
104]. The setting and hardening speed is faster than cement slurry, and the 60 day average strength of the filled body reached 3.24 MPa [
105]. Moreover, the cost was low, only about 20% of the same strength of cement filling materials, which made the RM filling material have a broad application prospect [
106].
The use of RM as cementitious filler could achieve high content utilization of RM, and produced filler could reach high strength and high flowability. However, it must consider heavy metal and radioactive elements in RM when use RM in cementitious filler. If these elements could not be solidified, serious second environmental pollution would occur. So, cheaper and/or effective solidifier was needed, and long-term environmental detection was also needed.