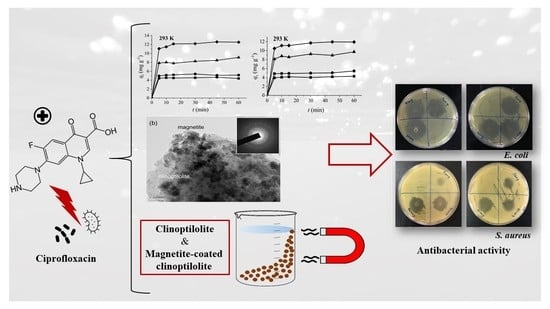
Use of Natural Clinoptilolite in the Preparation of an Efficient Adsorbent for Ciprofloxacin Removal from Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Magnetite
2.3. Preparation of Magnetite-Coated Clinoptilolite (MAG-CLI)
2.4. Characterization
2.4.1. Powder X-ray Diffraction Analysis (PXRD)
2.4.2. Energy Dispersive X-ray Spectroscopy (EDS)
2.4.3. Thermal Analysis
2.4.4. Textural Properties
2.4.5. TEM Analysis
2.4.6. FTIR Analysis
2.4.7. Zeta Potential Measurement
2.4.8. Magnetic Measurements
2.5. CIP Adsorption Experiments
2.6. Leaching Test
2.7. Antibacterial Activity Test
3. Results and Discussion
3.1. Powder X-ray Diffraction (PXRD)
3.2. Energy Dispersive X-ray Spectroscopy (EDS)
3.3. Thermogravimetric Analysis (TGA)
3.4. TEM Analysis
3.5. FTIR Analysis
3.6. Zeta Potential Measurements
3.7. Textural Analysis
3.8. Magnetic Measurements
3.9. Adsorption Isotherm Study
Adsorption Mechanism
3.10. Kinetic Analysis
3.11. Leaching Test
3.12. Regeneration of the Adsorbent
3.13. Antibacterial Test
4. Conclusions
- Calcium-rich natural clinoptilolite shows a high adsorptive activity towards antibiotic ciprofloxacin;
- Clinoptilolite strongly adsorbs ciprofloxacin at a pH of 5 via electrostatic interactions and ion exchange reaction occurring between the ciprofloxacin cations and clinoptilolite;
- The adsorption proceeds quickly following the Lagergren’s pseudo-second order rate equation. More than 80% of the maximum adsorption capacity was achieved within the first 10 min for the temperature range of 283 to 293 K;
- Impregnation of clinoptilolite by nano-magnetite particles does not influence the adsorption ability and capacity of clinoptilolite, but brings magnetism to the clinoptilolite-based adsorbent which allows for the easy removal of the spent adsorbent by magnetic separation;
- Magnetite coverage protects the spent adsorbent from the CIP leaching through an interaction of the carboxylic groups of the adsorbed CIP and magnetite particles;
- Preliminary studies indicate that atmospheric pressure plasma could be an efficient method for the regeneration of spent adsorbent;
- Ciprofloxacin-containing clinoptilolite shows strong antibacterial activity towards pathogens (E. coli and S. aureus), suggesting its possible use in a tertiary stage of water treatment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment–A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Frade, V.M.F.; Dias, M.; Teixeira, A.C.S.C.; Palma, M.S.A. Environmental contamination by fluoroquinolones. Braz. J. Pharm. Sci. 2014, 50, 41–54. [Google Scholar] [CrossRef]
- Johnson, A.C.; Keller, V.; Dumont, E.; Sumpter, J.P. Assessing the concentrations and risks of toxicity from the antibiotics ciprofloxacin, sulfamethoxazole, trimethoprim and erythromycin in European rivers. Sci. Total Environ. 2015, 511, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Rakshit, S.; Sarkar, D.; Elzinga, E.J.; Punamiya, P.; Datta, R. Mechanisms of ciprofloxacin removal by nano-sized magnetite. J. Hazard. Mater. 2013, 246–247, 221–226. [Google Scholar] [CrossRef]
- Genç, N.; Dogan, E.C. Adsorption kinetics of the antibiotic ciprofloxacin on bentonite, activated carbon, zeolite, and pumice. Desalin. Water Treat. 2013, 53, 1–9. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Thavorn-Amornsri, T.; Pereira, M.F.R.; Serp, P.; Figueiredo, J.L. Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal. Today 2012, 186, 29–34. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced oxidation processes for the removal of antibiotic from water. An overview. Water 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- De Witte, B.; Van Langenhove, H.; Demeestere, K.; Saerens, K.; De Wispelaere, P.; Dewilf, J. Ciprofloxacin ozonation in hospital wastewater treatment plant effluent: Effect of pH and H2O2. Chemosphere 2010, 78, 1142–1147. [Google Scholar] [CrossRef]
- Li, W.; Guo, C.; Su, B.; Xu, J. Photodegradation of four fluoroquinolone compounds by titanium dioxide under simulated solar light irradiation. J. Chem. Technol. Biotechnol. 2011, 87, 643–650. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Biodegradation and adsorption of antibiotics in the activated sludge process. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar] [CrossRef]
- Dorival-García, N.; Zafra-Gómez, A.; Navalón, A.; González, J.; Vílchez, J.L. Removal of quinolone antibiotics from wastewater by sorption and biological degradation in laboratory-scale membrane bioreactors. Sci. Total Environ. 2013, 442, 317–328. [Google Scholar] [CrossRef]
- Jia, A.; Wan, Y.; Xiao, Y.; Hu, J. Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res. 2012, 46, 387–394. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lee, C.-Y. Adsorption of ciprofloxacin in water using Fe3O4 nanoparticles formed at low temperature and high reactant concentrations in a rotating packed bed with co-precipitation. Mater. Chem. Phys. 2020, 240, 122049. [Google Scholar] [CrossRef]
- Attia, T.M.S.; Hu, X.L.; Qiang, Y.D. Synthesized magnetic nanoparticles coated zeolite for the adsorption of pharmaceutical compounds from aqueous solution using batch and column studies. Chemosphere 2013, 93, 2076–2085. [Google Scholar] [CrossRef]
- Hsini, A.; Essekri, A.; Aarab, N.; Laabd, M.; Addi, A.A.; Lakhmiri, R.; Albourine, A. Elaboration of novel polyaniline@Almond shell biocomposite for effective removal of hexavalent chromium ions and Orange G dye from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 15245–15258. [Google Scholar] [CrossRef]
- Hsini, A.; Naciri, Y.; Benafqir, M.; Ajmal, Z.; Aarab, N.; Laabd, M.; Navío, J.A.; Puga, F.; Boukherroub, R.; Bakiz, B.; et al. Facile synthesis and characterization of a novel 1,2,4,5-benzene tetracarboxylic acid doped polyaniline@zinc phosphate nanocomposite for highly efficient removal of hazardous hexavalent chromium ions from water. J. Colloid Interface Sci. 2021, 585, 560–573. [Google Scholar] [CrossRef]
- Hsini, A.; Naciri, Y.; Laabd, M.; El Ouardi, M.; Ajmal, Z.; Lakhmiri, R.; Boukherroub, R.; Albourine, A. Synthesis and characterization of arginine-doped polyaniline/walnut shell hybrid composite with superior clean-up ability for chromium (VI) from aqueous media: Equilibrium, reusability and process optimization. J. Mol. Liq. 2020, 316, 113832. [Google Scholar] [CrossRef]
- Ma, J.; Yang, M.; Yu, F.; Zheng, J. Water-enhanced removal of ciprofloxacin from water by porous graphene hydrogel. Sci. Rep. 2015, 5, 13578. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Yang, M.; Yu, F.; Ma, J. Response surface methodological evaluation and optimization for adsorption removal of ciprofloxacin onto graphene hydrogel. J. Mol. Liq. 2019, 284, 124–130. [Google Scholar] [CrossRef]
- Nogueira, J.; António, M.; Mikhalev, S.M.; Fateixa, S.; Trindade, T.; Daniel-da-Silva, L.A. Porous carrageenan-derived carbon for efficient ciprofloxacin removal from water. J. Nanomater. 2018, 8, 1004. [Google Scholar] [CrossRef] [Green Version]
- El-Shafey, E.-S.I.; Al-Lawati, H.; Al-Sumari, A.S. Ciprofloxacin adsorption from aqueous solution onto chemically prepared carbon from date palm leaflets. J. Environ. Sci. 2012, 24, 1579–1586. [Google Scholar] [CrossRef]
- Cheng, R.; Li, H.; Liu, Z.; Du, C. Halloysite nanotubes as an effective and recyclable adsorbent for removal of low-concentration antibiotics ciprofloxacin. Minerals 2018, 8, 387. [Google Scholar] [CrossRef] [Green Version]
- Najafpoor, A.A.; Sani, O.N.; Alidadi, H.; Yazdani, M.; Fezabady, A.A.N.; Taghavi, M. Optimization of ciprofloxacin adsorption from synthetic wastewater using γ-Al2O3 nanoparticles: An experimental design based on response surface methodology. Colloids Interface Sci. Commun. 2019, 33, 100212. [Google Scholar] [CrossRef]
- Fan, H.; Ma, Y.; Wan, J.; Wang, Y.; Li, Z.; Chen, Y. Adsorption properties and mechanisms of novel biomaterials from banyan aerial roots via simple modification for ciprofloxacin removal. Sci. Total Environ. 2020, 708, 134630. [Google Scholar] [CrossRef]
- Ashiq, A.; Sarkar, B.; Adassooriya, N.; Walpita, J.; Rajapaksha, A.U.; Ok, Y.S.; Vithanage, M. Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 2019, 236, 124384. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Qiao, G.-L.; Zhao, F.; Wang, Y. Thermodynamic and kinetic parameters of ciprofloxacin adsorption onto modified coal fly ash from aqueous solution. J. Mol. Liq. 2011, 163, 53–56. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Ngeno, E.C.; Shikuku, V.O.; Orata, F.; Baraza, L.D.; Kimosop, S.J. Caffeine and ciprofloxacin adsorption from water onto clinoptilolite: Linear isotherms, kinetics, thermodynamic and mechanistic studies. S. Afr. J. Chem. 2019, 72, 139–142. [Google Scholar] [CrossRef]
- Zide, D.; Fatoki, O.; Oputu, O.; Opeolu, B.; Nelana, S.; Olatunji, O. Zeolite ‘adsorption’ capacities in aqueous acidic media; The role of acid choice and quantification method on ciprofloxacin removal. Microporous Mesoporous Mater. 2018, 255, 226–241. [Google Scholar] [CrossRef]
- Wang, C.-J.; Li, Z.; Jiang, W.-T.; Jean, J.-S.; Liu, C.-C. Cation exchange interaction between antibiotic ciprofloxacin and montmorillonite. J. Hazard. Mater. 2010, 183, 309–314. [Google Scholar] [CrossRef]
- Rajić, N.; Stojaković, D.; Jovanović, M.; Zabukovec Logar, N.; Mazaj, M.; Kaučič, V. Removal of nickle(II) ions from aqueous solutions using the natural clinoptilolite and preparation of nano-NiO on the exhausted clinoptilolite. Appl. Surf. Sci. 2010, 257, 1524–1532. [Google Scholar] [CrossRef]
- Stojaković, D.; Milenković, J.; Daneu, N.; Rajić, N. A study of the removal of copper ions from aqueous solution using clinoptilolite from Serbia. Clay Clay Miner. 2012, 59, 277–285. [Google Scholar] [CrossRef]
- Pavlović, J.B.; Milenković, J.K.; Rajić, N.Z. Modification of natural clinoptilolite for nitrate removal from aqueous media. J. Serb. Chem. Soc. 2014, 79, 1309–1322. [Google Scholar] [CrossRef]
- Jevtić, S.; Arčon, I.; Rečnik, A.; Babić, B.; Mazaj, M.; Pavlović, J.; Matijašević, D.; Nikšić, M.; Rajić, N. The iron(III)-modified natural zeolitic tuff as an adsorbent and carrier for selenium oxyanions. Microporous Mesoporous Mater. 2014, 197, 92–100. [Google Scholar] [CrossRef]
- Muir, B.; Wołowiec, M.; Bajda, T.; Nowak, P.; Czupryński, P. The removal of organic compounds by natural and synthetic surface-functionalized zeolites: A mini-review. Mineralogia 2017, 48, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Ambrozova, P.; Kynicky, J.; Urubek, T.; Nguyen, V.D. Synthesis and modification of clinoptilolite. Molecules 2017, 22, 1107. [Google Scholar] [CrossRef] [Green Version]
- Rajic, N.; Stojakovic, D.; Daneu, N.; Recnik, A. The formation of oxide nanoparticles on the surface of natural clinoptilolite. J. Phys. Chem. Solids 2011, 72, 800–803. [Google Scholar] [CrossRef]
- Ahribesh, A.A.; Lazarević, S.; Janković-Častvan, I.; Jokić, B.; Spasojević, V.; Radetić, T.; Janaćković, Đ.; Petrović, R. Influence of the synthesis parameters on the properties of the sepiolite-based magnetic adsorbents. Powder Technol. 2017, 305, 260–269. [Google Scholar] [CrossRef]
- Javanbakht, V.; Ghoreishi, S.M.; Habibi, N.; Javanbakht, M. A novel magnetic chitosan/clinoptilolite/magnetite nanocomposite for highly efficient removal of Pb(II) ions from aqueous solution. Powder Technol. 2016, 302, 372–383. [Google Scholar] [CrossRef]
- Savić, A.B.; Čokeša, D.; Lazarević, S.; Jokić, B.; Janaćković, D.; Petrović, R.; Živković, L.S. Tailoring of magnetic powder properties for enhanced phosphate removal: Effect of PEG addition in the synthesis process. Powder Technol. 2016, 301, 511–519. [Google Scholar] [CrossRef]
- Arora, M.; Eddy, N.K.; Mumford, K.A.; Baba, Y.; Perera, J.M.; Stevens, G.W. Surface modification of natural zeolite by chitosan and its use for nitrate removal in cold regions. Cold. Reg. Sci. Technol. 2010, 62, 92–97. [Google Scholar] [CrossRef]
- Kragović, M.; Pašalić, S.; Marković, M.; Petrović, M.; Nedeljković, B.; Momčilović, M.; Stojmenović, M. Natural and modified zeolite–alginate composites. Application for removal of heavy metal cations from contaminated water solutions. Minerals 2017, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Braschi, I.; Blasioli, S.; Gigli, L.; Gessa, C.E.; Alberti, A.; Martucci, A. Removal of sulfonamide antibiotics from water: Evidence of adsorption into an organophilic zeolite Y by its structural modifications. J. Hazard. Mater. 2010, 178, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A. TOPAS Academic 4.1; Coelho Software: Brisbane, Australia, 2007. [Google Scholar]
- Ming, D.W.; Dixon, J.B. Quantitative determination of clinoptilolite in soils by a cation-exchange capacity method. Clay Clay Miner. 1987, 35, 463–468. [Google Scholar] [CrossRef]
- Khalil, M.I. Co-precipitation in aqueous solution synthesis of magnetite nanoparticles using iron(III) salts as precursors. Arab. J. Chem. 2015, 8, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Mohseni-Bandpi, A.; Al-Musawi, T.J.; Ghahramani, E.; Zarrabi, M.; Mohebi, S.; Vahed, S.A. Improvement of zeolite adsorption capacity for cephalexin by coating with magnetic Fe3O4 nanoparticles. J. Mol. Liq. 2016, 218, 615–624. [Google Scholar] [CrossRef]
- Gulicovski, J.J.; Čerović, L.S.; Milonjić, S.K. Point of zero charge and isoelectric point of alumina. Mater. Manuf. Process. 2008, 23, 615–619. [Google Scholar] [CrossRef]
- Naveed, S.; Waheed, N. Simple UV spectrophotometric assay of ciprofloxacin. MJPMS 2014, 3, 10–13. [Google Scholar]
- Hawash, H.B.I.; Chmielewska, E.; Netriová, Z.; Majzlan, J.; Pálková, H.; Sokolík, R. Innovative comparable study for application of iron oxyhydroxide and manganese dioxide modified clinoptilolite in removal of Zn(II) from aqueous medium. J. Environ. Chem. Eng. 2018, 6, 6489–6503. [Google Scholar] [CrossRef]
- Rajput, S.; Pittman, C.U., Jr.; Mohan, D. Magnetic magnetite (Fe3O4) nanoparticles synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Kouli, M.-E.; Banis, G.; Tsarabaris, P.; Ferraro, A.; Hristoforou, E. A study on magnetic removal of sodium, calcium and potassium ions from seawater using magnetite/clinoptilolite–Na composite nanoparticles. J. Magn. Magn. Mater. 2018, 465, 692–699. [Google Scholar] [CrossRef]
- Attia, T.M.S.; Hu, X.L.; Yin, D.Q. Synthesised magnetic nanoparticles coated zeolite (MNCZ) for the removal of arsenic (As) from aqueous solution. J. Exp. Nanosci. 2014, 9, 551–560. [Google Scholar] [CrossRef]
- Rečnik, A.; Nyirő-Kósa, I.; Dódony, I.; Pósfai, M. Growth defects and epitaxy in Fe3O4 and γ-Fe2O3 nanocrystals. Cryst. Eng. Comm. 2013, 15, 7539–7547. [Google Scholar] [CrossRef]
- Nyirő-Kósa, I.; Rečnik, A.; Pósfai, M. Novel methods for the synthesis of magnetite nanoparticles with special morphologies and textured assemblages. J. Nanopart. Res. 2012, 14, 1150–1159. [Google Scholar] [CrossRef]
- Jordan, V.; Javornik, U.; Plavec, J.; Podgornik, A.; Rečnik, A. Self-assembly of multilevel branched rutile-type TiO2 structures via oriented lateral and twin attachment. Sci. Rep. 2016, 6, 24216. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Q.-L.; Dong, Y.-B.; He, Y.-H.; Wang, L. Physicochemical properties and mechanism study of clinoptilolite modified by NaOH. Microporous Mesoporous Mater. 2015, 218, 174–179. [Google Scholar] [CrossRef]
- Cotton, A. Dissolution kinetics of clinoptilolite and heulandite in alkaline conditions. Biosci. Horiz. 2008, 1, 38–43. [Google Scholar] [CrossRef]
- Duan, W.; Wang, N.; Xiao, W.; Zhao, Y.; Zheng, Y. Ciprofloxacin adsorption onto different micro-structured tourmaline, halloysite and biotite. J. Mol. Liq. 2018, 269, 874–881. [Google Scholar] [CrossRef]
- Wang, F.; Yang, B.; Wang, H.; Song, Q.; Tan, F.; Cao, Y. Removal of ciprofloxacin from aqueous solution by a magnetic chitosan grafted graphene oxide composite. J. Mol. Liq. 2016, 222, 188–194. [Google Scholar] [CrossRef]
- Ullah, S.; Azmi, B.M.; Ali, A.M.; Al-Sehemi, A.G.; Gonfa, G.; Mukhtar, A.; Abdul Kareem, F.A.; Ayoub, M.; Saqib, S.; Binti Mellon, N. Synthesis and characterization of mesoporous MOF UMCM-1 for CO2/CH4 adsorption; an experimental, isotherm modeling and thermodynamic study. Microporous Mesoporous Mat. 2020, 294, 1387–1811. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica, and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Si, Y.; Wang, F.; Su, L.; Xia, C.; Yao, J.; Chen, H.; Liu, X. Interaction processes of ciprofloxacin with graphene oxide and reduce graphene oxide in the presence of montmorillonite in simulated gastrointestinal fluids. Sci. Rep. 2017, 7, 2588. [Google Scholar]
- Weber, W.J., Jr.; Morris, J.C. Advances in Water Pollution Research: Removal of biologically resistant pollutants from waste waters by adsorption. In Proceedings of the International Conference on Water Pollution Symposium; Pergamon Press: London, UK, 1962. [Google Scholar]
- Mirzaei, H.; Almasian, M.R.; Mousavian, S.M.A.; Kalal, H.S. Plasma modification of a natural zeolite to improve its adsorption capacity of strontium ions from water samples. Int. J. Environ. Sci. Technol. 2019, 16, 6157–6166. [Google Scholar] [CrossRef]
- Garcia, J.J.M.; Nuñez, J.A.P.; Salapare, H.S.; Vasquez, M.R., Jr. Adsorption of diclofenac sodium in aqueous solution using plasma-activated natural zeolites. Results Phys. 2019, 15, 102629. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Proc. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Hrenovic, J.; Milenkovic, J.; Ivankovic, T.; Rajic, N. Antibacterial activity of heavy metal-loaded natural zeolite. J. Hazard. Mater. 2012, 201–202, 260–264. [Google Scholar] [CrossRef]
- Simões, M.; Rocha, S.; Coimbra, M.; Vieira, M. Enhancement of Escherichia coli and Staphylococcus aureus antibiotic susceptibility using sesquiterpenoids. J. Med. Chem. 2008, 4, 616–623. [Google Scholar] [CrossRef] [Green Version]
- Chalkley, L.J.; Koornhof, H.J. Antimicrobial activity of ciprofloxacin against Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus determined by the killing curve method: Antibiotic comparisons and synergistic interactions. Antimicrob. Agents Chemother. 1985, 28, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Berlanga, M.; Montero, M.T.; Hernández-Borrell, J.; Viñas, M. Influence of the cell wall on ciprofloxacin susceptibility in selected wild-type Gram-negative and Gram-positive bacteria. Int. J. Antimicrob. Agents 2004, 23, 627–630. [Google Scholar] [CrossRef]
- Mohsen, E.; El-Borady, O.M.; Mohamed, M.B.; Fahim, I.S. Synthesis and characterization of ciprofloxacin loaded silver nanoparticles and investigation of their antibacterial effect. J. Radiat. Res. Appl. 2020, 13, 416–425. [Google Scholar] [CrossRef] [Green Version]














| Adsorbent | Experimental Conditions | CIP Removal Efficiency, % | Recyclability | Ref. |
|---|---|---|---|---|
| Nano-sized magnetite | C0 = 33 mg dm−3 Adsorbent dose = 10 g dm−3 pH = 5.97 t = 24 h | 80 | * n.r. | [5] |
| Graphene hydrogel | C0 = 50 mg dm−3 T = 25 °C t ≈ 36 h | 75 | n.r. | [19] |
| Carbon from date palm leaflets | C0 = 100 mg dm−3 Adsorbent dose = 2 g dm−3 pH = 6 T = 45 °C t = 48 h | 56 | n.r. | [22] |
| Halloysite nanotubes | C0 = 30 mg dm−3 Adsorbent dose = 1.7 g dm−3 pH = 5–6 T = 20 °C t = 90 min | 95 | 95% after five cycles | [23] |
| γ-Al2O3 nanoparticles | C0 = 20 mg dm−3 Adsorbent dose = 0.775 g dm−3 pH = 7.5 t = 46.25 min | 53 | n.r. | [24] |
| Biomaterials from banyan aerial roots | C0 = 60 mg dm−3 Adsorbent dose = 1.2 g dm−3 pH = 8 T = 25 °C t = 48 h | 97 | n.r. | [25] |
| Biochar-montmorillonite | C0 = 25 mg dm−3 Adsorbent dose = 1 g dm−3 pH = 5–6 t = 400 min | 86 | n.r. | [26] |
| Coal fly ash | C0 = 160 mg dm−3 Adsorbent dose = 40 g dm−3 T = 40 °C t = 100 min | 39 | n.r. | [27] |
| Clinoptilolite | C0 = 5 mg dm−3 Adsorbent dose = 2 g dm−3 pH = 6 T = 25 °C | 99 | n.r. | [29] |
| Synthesized zeolites (A, X, Y) | C0 = 150 mg dm−3 Adsorbent dose = 0.5 g dm−3 pH = 3 T = 20 °C t = 24 h | 27–61.4 | n.r. | [30] |
| Commercial zeolites (A, X, Y) | C0 = 150 mg dm−3 Adsorbent dose = 0.5 g dm−3 pH = 3 T = 20 °C t = 24 h | 34–87 | n.r. | [30] |
| Sample | Si | Al | O | K | Ca | Fe | Si/Al |
|---|---|---|---|---|---|---|---|
| at.% | |||||||
| CLI | 18.35 | 3.65 | 75.97 | 0.44 | 1.20 | 0.40 | 5.03 |
| MAG-CLI | 18.39 | 3.01 | 72.15 | 0.27 | 0.18 | 5.63 | 6.12 |
| Sample | SBET (m2 g−1) | Vtot (cm3 g−1) | Dmax (nm) |
|---|---|---|---|
| CLI | 23.57 | 0.0988 | 16.26 |
| MAG-CLI | 45.17 | 0.1531 | 3.50 |
| CLI | ||||||
| T, K | Langmuir Isotherm | Freudlich Isotherm | ||||
| qmax, mg g−1 | bL, dm3 mg−1 | R2 | Kf, mg g−1 (dm3 mg−1)1/n | n | R2 | |
| 283 | 14.96 | 0.1028 | 0.9944 | 2.24 | 2.05 | 0.9924 |
| 288 | 16.31 | 0.0697 | 0.9877 | 1.73 | 1.80 | 0.9804 |
| 293 | 17.30 | 0.0893 | 0.9909 | 2.34 | 1.97 | 0.9993 |
| MAG-CLI | ||||||
| T, K | Langmuir Isotherm | Freundlich Isotherm | ||||
| qmax, mg g−1 | bL, dm3 mg−1 | R2 | Kf, mg g−1 (dm3 mg−1)1/n | n | R2 | |
| 283 | 13.27 | 0.2235 | 0.9834 | 3.34 | 2.62 | 0.9831 |
| 288 | 15.86 | 0.0914 | 0.9827 | 2.16 | 1.97 | 0.9809 |
| 293 | 14.25 | 0.2263 | 0.9870 | 3.85 | 2.81 | 0.9981 |
| Sample | Cations | ||
|---|---|---|---|
| K+ | Mg2+ | Ca2+ | |
| mg dm−3 | |||
| CLI | 0.9325 | 1.2513 | 4.2280 |
| MAG-CLI | 0.6796 | 0.3823 | 2.1890 |
| CLI | |||||||
| C0, mg CIP dm−3 | T, K | Weber-Morris Model Parameters | Lagergren’s Pseudo-Second-Order Rate Parameters | ||||
| Kid, mg g−1 min−1/2 | I, mg g−1 | R2 | k2, g mg−1 min−1 | qe, mg g−1 | R2 | ||
| 15 | 283 | 0.0729 | 3.54 | 0.9301 | 0.3393 | 4.09 | 0.9999 |
| 288 | 0.0768 | 3.66 | 0.9638 | 0.2490 | 4.28 | 0.9998 | |
| 293 | 0.0599 | 4.29 | 0.9221 | 0.2275 | 4.82 | 0.9996 | |
| 25 | 283 | 0.0592 | 4.35 | 0.7707 | 0.1896 | 4.90 | 0.9988 |
| 288 | 0.0820 | 4.50 | 0.8933 | 0.2900 | 5.13 | 0.9999 | |
| 293 | 0.0377 | 4.99 | 0.5450 | 0.4011 | 5.24 | 0.9995 | |
| 50 | 283 | 0.3033 | 7.15 | 0.9436 | 0.0436 | 9.78 | 0.9983 |
| 288 | 0.1946 | 6.93 | 0.9386 | 0.0703 | 8.63 | 0.9991 | |
| 293 | 0.1632 | 7.44 | 0.8792 | 0.0714 | 8.94 | 0.9989 | |
| 75 | 283 | 0.1442 | 10.26 | 0.7653 | 0.0868 | 11.58 | 0.9997 |
| 288 | 0.1822 | 9.77 | 0.7612 | 0.0886 | 11.28 | 0.9991 | |
| 293 | 0.3307 | 10.46 | 0.9285 | 0.0443 | 13.27 | 0.9982 | |
| MAG-CLI | |||||||
| C0, mg CIP dm−3 | T, K | Weber-Morris Model Parameters | Lagergren’s Pseudo-Second-Order Rate Parameters | ||||
| Kid, mg g−1 min−1/2 | I, mg g−1 | R2 | k2, g mg−1 min−1 | qe, mg g−1 | R2 | ||
| 15 | 283 | 0.0390 | 3.07 | 0.7268 | 0.3133 | 3.43 | 0.9997 |
| 288 | 0.0877 | 2.24 | 0.9268 | 0.1361 | 3.03 | 0.9964 | |
| 293 | 0.0434 | 3.88 | 0.7702 | 0.3242 | 4.26 | 0.9995 | |
| 25 | 283 | 0.1337 | 4.46 | 0.9771 | 0.1159 | 5.59 | 0.9996 |
| 288 | 0.0399 | 4.88 | 0.4335 | 0.1185 | 5.08 | 0.9996 | |
| 293 | 0.0885 | 4.59 | 0.8507 | 0.1257 | 5.41 | 0.9980 | |
| 50 | 283 | 0.1972 | 7.29 | 0.9393 | 0.1359 | 8.60 | 0.9995 |
| 288 | 0.1708 | 6.38 | 0.9613 | 0.0861 | 7.84 | 0.9993 | |
| 293 | 0.1060 | 8.21 | 0.5944 | 0.0673 | 8.85 | 0.9994 | |
| 75 | 283 | 0.3857 | 9.59 | 0.7299 | 0.0346 | 13.00 | 0.9930 |
| 288 | 0.2409 | 10.12 | 0.8105 | 0.0859 | 12.00 | 0.9994 | |
| 293 | 0.3047 | 9.96 | 0.9832 | 0.0558 | 12.46 | 0.9997 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalebić, B.; Pavlović, J.; Dikić, J.; Rečnik, A.; Gyergyek, S.; Škoro, N.; Rajić, N. Use of Natural Clinoptilolite in the Preparation of an Efficient Adsorbent for Ciprofloxacin Removal from Aqueous Media. Minerals 2021, 11, 518. https://0-doi-org.brum.beds.ac.uk/10.3390/min11050518
Kalebić B, Pavlović J, Dikić J, Rečnik A, Gyergyek S, Škoro N, Rajić N. Use of Natural Clinoptilolite in the Preparation of an Efficient Adsorbent for Ciprofloxacin Removal from Aqueous Media. Minerals. 2021; 11(5):518. https://0-doi-org.brum.beds.ac.uk/10.3390/min11050518
Chicago/Turabian StyleKalebić, Barbara, Jelena Pavlović, Jelena Dikić, Aleksander Rečnik, Sašo Gyergyek, Nikola Škoro, and Nevenka Rajić. 2021. "Use of Natural Clinoptilolite in the Preparation of an Efficient Adsorbent for Ciprofloxacin Removal from Aqueous Media" Minerals 11, no. 5: 518. https://0-doi-org.brum.beds.ac.uk/10.3390/min11050518