Phenolic Glycosides from Capsella bursa-pastoris (L.) Medik and Their Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results and Discussion
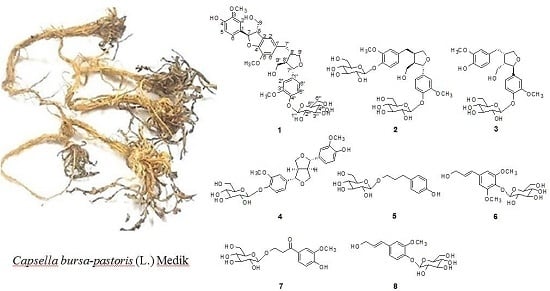
2.1. Structure Elucidation
2.2. Anti-Inflammatory Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Characterization
3.5. Enzymatic Hydrolysis
3.6. Measurement of Nitric Oxide Production and Cell Viability in LPS-Activated BV-2 Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, Y.N. Flora of Korea; Kim, S., Ed.; Kyohaksa: Seoul, Korea, 1998; p. 259. [Google Scholar]
- Kuroda, K.; Kaku, T. Pharmacological and chemical studies on the alcohol extract of Capsella bursa-pastoris. Life Sci. 1969, 8, 151–155. [Google Scholar] [CrossRef]
- Song, N.; Xu, W.; Guan, H.; Liu, X.; Wang, Y.; Nie, X. Several flavonoids from Capsella bursa-pastoris (L.) Medic. Asian J. Tradit. Med. 2007, 2, 218–222. [Google Scholar]
- Grosso, C.; Vinholes, J.; Silva, L.R.; Pinho, P.G.d.; Gonçalves, R.F.; Valentão, P.; Jäger, A.K.; Andrade, P.B. Chemical composition and biological screening of Capsella bursa-pastoris. Rev. Bras. Farmacogn. 2011, 21, 635–643. [Google Scholar] [CrossRef]
- Selenu, M.; Carrus, F.; Bonsignore, L. Phytochemical study on capsella bursa pastoris l. Boll. Chim. Farm. 2005, 144, 66–78. [Google Scholar]
- Park, C.J.; Park, C.B.; Hong, S.S.; Lee, H.S.; Lee, S.Y.; Kim, S.C. Characterization and cdna cloning of two glycine-and histidine-rich antimicrobial peptides from the roots of shepherd’s purse, Capsella bursa-pastoris. Plant Mol. Biol. 2000, 44, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Akao, M. Antitumor and anti-intoxication activities of fumaric acid in cultured cells. Gann 1981, 72, 777–782. [Google Scholar] [PubMed]
- Kuroda, K.; Mitsutaro, A. Effect of Capsella bursa-pastoris on liver catalase activity in rats fed 3′-methyl-4-(dimethylamino) azobenzene. Gann 1975, 66, 461–462. [Google Scholar] [PubMed]
- Hur, J.; Yoo, M.; Shin, D.B.; Lee, S. Inhibition of nitric oxide production corresponds to the sulforaphane content in Korean sheperd’s purse (Capsella bursa-pastoris) and related species in BV-2 cell. Food Sci. Biotechnol. 2013, 22, 1085–1089. [Google Scholar] [CrossRef]
- El Gamal, A.; Takeya, K.; Itokawa, H.; Halim, A.; Amer, M.; Saad, H.E. Lignan bis-glucosides from Galium sinaicum. Phytochemistry 1997, 45, 597–600. [Google Scholar] [CrossRef]
- Karioti, A.; Protopappa, A.; Megoulas, N.; Skaltsa, H. Identification of tyrosinase inhibitors from Marrubium velutinum and Marrubium cylleneum. Bioorg. Med. Chem. 2007, 15, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lim, J.P.; Kim, J.W.; Park, H.W.; Eun, J.S. Antitumor and antiinflammatory constituents from Celtis sinensis. Arch. Pharm. Res. 2005, 28, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Akita, H.; Kawahara, E.; Kishida, M.; Kato, K. Synthesis of naturally occurring β-D-glucopyranoside based on enzymatic β-glycosidation. J. Mol. Catal. B: Enzym. 2006, 40, 8–15. [Google Scholar] [CrossRef]
- Della Greca, M.; Ferrara, M.; Fiorentino, A.; Monaco, P.; Previtera, L. Antialgal compounds from Zantedeschia aethiopica. Phytochemistry 1998, 49, 1299–1304. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, Y.S.; Sa, Y.J.; Kim, M.J. Isolation and identification of sea buckthorn (Hippophae rhamnoides) phenolics with antioxidant activity and α-glucosidase inhibitory effect. J. Agric. Food Chem. 2010, 59, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Yang, X.W.; Zhang, M.; Zhong, G.Y. Phytochemical study of the rhizome of Pinellia ternata and quantification of phenylpropanoids in commercial Pinellia tuber by RP-LC. Chromatographia 2006, 64, 647–653. [Google Scholar] [CrossRef]
- Ozawa, S.; Sasaya, T. Extractives of Todomatsu Abies sachalinensis Masters, 7: New phenylpropane trimers from the wood of Abies sachalinensis. Jpn. Wood Res. Soc. 1991, 37, 69–75. [Google Scholar]
- Perkins, S.J.; Johnson, L.N.; Phillips, D.C.; Dwek, R.A. High resolution 1H-and 13N-NMR spectra of d-gluco-pyranose, 2-acetamido-2-deoxy-d-glucopyranose, and related compounds in aqueous media. Carbohydr. Res. 1977, 59, 19–34. [Google Scholar] [CrossRef]
- Yang, Y.N.; Huang, X.Y.; Feng, Z.M.; Jiang, J.S.; Zhang, P.C. Hepatoprotective activity of twelve novel 7′-hydroxy lignan glucosides from Arctii fructus. J. Agric. Food Chem. 2014, 62, 9095–9102. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Choi, J.W.; Choi, S.U.; Lee, K.R. Terpene glycosides and cytotoxic constituents from the seeds of Amomum xanthioides. Planta Med. 2010, 76, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res. Ther. 2008, 10, S2. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Gaire, B.P.; Do, M.H.; Lee, T.H.; Kim, S.Y. Anti-neuroinflammatory and neuroprotective effects of the Lindera neesiana fruit in vitro. Phytomedicine 2016, 23, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Sohn, J.H.; Kim, Y.C.; Oh, H. Viridicatol from the marine-derived fungal strain Penicillium sp. SF-5295 exerts anti-inflammatory effects through inhibiting NF-ΚB signaling pathway on lipopolysaccharide-induced RAW264. 7 and BV2 cells. Nat. Prod. Sci. 2015, 21, 240–247. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Position | δC, Type | δH (J in Hz) |
|---|---|---|
| 1 | 135.7, C | |
| 2 | 111.3, CH | 7.05, d (1.7) |
| 3 | 147.8, C | |
| 4 | 147.5, C | |
| 5 | 118.1, CH | 7.15, d (8.2) |
| 6 | 119.5, CH | 6.95, dd (8.2, 1.7) |
| 7 | 88.6, CH | 5.58, d (5.91) |
| 8 | 55.7, CH | 3.48, m |
| 9 | 65.1, CH2 | |
| a | 3.78, m | |
| b | 3.85, m | |
| 1′ | 134.9, C | |
| 2′ | 114.7, CH | 6.78, s |
| 3′ | 147.9, C | |
| 4′ | 147.9, C | |
| 5′ | 145.5, C | |
| 6′ | 118.4, CH | 6.76, s |
| 7′ | 33.9, CH2 | |
| a | 2.96, dd (13.5, 5.0) | |
| b | 2.57, dd (13.3, 11.0) | |
| 8′ | 44.0, CH | 2.75, sep (6.5, 5.5) |
| 9′ | 73.5, CH2 | |
| a | 3.78, m | |
| b | 4.05, dd (8.3, 6.6) | |
| 1″ | 139.7, C | |
| 2″ | 111.5, CH | 7.01, d (1.7) |
| 3″ | 151.1, C | |
| 4″ | 147.5, C | |
| 5″ | 118.2, CH | 7.16, d (8.3) |
| 6″ | 119.7, CH | 6.90, dd (8.2, 1.7) |
| 7″ | 83.9, CH | 4.85, overlap |
| 8″ | 54.2, CH | 2.38, quin (6.9) |
| 9″ | 60.5, CH2 | |
| a | 3.88, m | |
| b | 3.68, dd (10.9, 6.7) | |
| Glc-1′′′ | 103.1, CH | 4.91, d (7.3) |
| 2′′′ | 75.1, CH | 3.51, m |
| 3′′′ | 78.5, CH | 3.39, m |
| 4′′′ | 71.6, CH | 3.41, m |
| 5′′′ | 78.4, CH | 3.41, m |
| 6′′′ | 62.6, CH | 3.69, overlap |
| OCH3 (3″) | 56.9 | 3.84, s |
| OCH3 (3) | 56.8 | 3.88, s |
| OCH3 (5′) | 56.8 | 3.87, s |
| Compound | IC50 a (µM) | Cell Viability b (%) |
|---|---|---|
| 1 | 75.13 | 103.04 ± 7.08 |
| 2 | 48.80 | 116.68 ± 3.69 |
| 3 | 30.70 | 109.17 ± 8.61 |
| 4 | 17.80 | 117.36 ± 10.23 |
| 5 | 31.14 | 116.09 ± 10.67 |
| 6 | 62.21 | 110.02 ± 9.52 |
| 7 | 27.91 | 119.20 ± 6.23 |
| 8 | 49.21 | 111.55 ± 10.40 |
| c L-NMMA | 20.76 | 112.89 ± 4.90 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, J.M.; Suh, W.S.; Lee, T.H.; Subedi, L.; Kim, S.Y.; Lee, K.R. Phenolic Glycosides from Capsella bursa-pastoris (L.) Medik and Their Anti-Inflammatory Activity. Molecules 2017, 22, 1023. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22061023
Cha JM, Suh WS, Lee TH, Subedi L, Kim SY, Lee KR. Phenolic Glycosides from Capsella bursa-pastoris (L.) Medik and Their Anti-Inflammatory Activity. Molecules. 2017; 22(6):1023. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22061023
Chicago/Turabian StyleCha, Joon Min, Won Se Suh, Tae Hyun Lee, Lalita Subedi, Sun Yeou Kim, and Kang Ro Lee. 2017. "Phenolic Glycosides from Capsella bursa-pastoris (L.) Medik and Their Anti-Inflammatory Activity" Molecules 22, no. 6: 1023. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22061023