Magnetic Solid-phase Extraction with Fe3O4/Molecularly Imprinted Polymers Modified by Deep Eutectic Solvents and Ionic Liquids for the Rapid Purification of Alkaloid Isomers (Theobromine and Theophylline) from Green Tea
Abstract
:1. Introduction
2. Results and Discussion
2.1. Validation of MSPE-HPLC Method
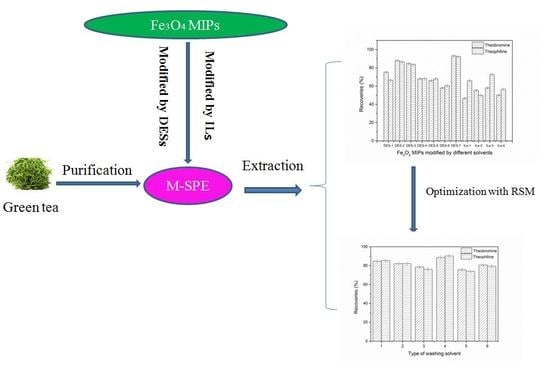
2.2. Purification of the Extracts with Solid-Phase Extraction
2.3. Characterizations
2.4. Adsorption Properties of Fe3O4/MIPs and DESs-7-Fe3O4/MIPs
2.5. Optimization of M-SPE Procedure
2.6. Analysis of Green Tea Samples
3. Experimental
3.1. Reagents and Materials
3.2. Chromatography and Sample Pretreatment
3.3. Preparation of Fe3O4/MIPs and DESs (ILs)-Fe3O4/MIPs
3.3.1. Preparation of DESs and ILs
3.3.2. Preparation of Fe3O4/MIPs
3.3.3. Preparation of DESs (ILs)-Fe3O4/MIPs
3.4. Characterization of the Fe3O4/MIPs and DESs (ILs)-Fe3O4/MIPs
3.5. Absorption Capacity of Fe3O4/MIPs and DESs-Fe3O4/MIPs
3.6. Purification of Theobromine and Theophylline from Green Tea by M-SPE
3.7. Optimization of M-SPE Procedure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sereshti, H.; Khosraviani, M.; Samadi, S.; Amini-Fazl, M.L. Simultaneous determination of theophylline, theobromine and caffeine in different tea beverages by graphene-oxide based ultrasonic-assisted dispersive micro solid-phase extraction combined with HPLC-UV. RSC Adv. 2014, 4, 47114–47120. [Google Scholar] [CrossRef]
- Liang, K.; Ng, S.; Lee, F.; Lim, J.; Chung, J.E.; Lee, S.S.; Kurisawa, M. Targeted intracellular protein delivery based on hyaluronic acid–green tea catechin nanogels. Acta Biomater. 2016, 33, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Damm, I.; Enger, E.; Chrubasik-Hausmann, S.; Schieber, A.; Zimmermann, B.F. Fast and comprehensive analysis of secondary metabolites in cocoa products using ultra high-performance liquid chromatography directly after pressurized liquid extraction. J. Sep. Sci. 2016, 39, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Mehari, B.; Redi-Abshiro, M.; Chandravanshi, B.S.; Atlabchew, M.; Combrinck, S.; McCrindle, R. Simultaneous determination of alkaloids in green coffee beans from Ethiopia: Chemometric evaluation of geographical origin. Food Anal. Methods 2016, 9, 1627–1637. [Google Scholar] [CrossRef]
- Caudle, A.G.; Gu, Y.; Bell, L.N. Improved analysis of theobromine and caffeine in chocolate food products formulated with cocoa powder. Food Res. Int. 2001, 34, 599–603. [Google Scholar] [CrossRef]
- Aresta, A.; Palmisano, F.; Zambonin, C.G. Simultaneous determination of caffeine, theobromine, theophylline, paraxanthine and nicotine in human milk by liquid chromatography with diode array UV detection. Food Chem. 2005, 93, 177–181. [Google Scholar] [CrossRef]
- Wang, L.; Xu, R.; Hu, B.; Li, W.; Sun, Y.; Tu, Y.Y.; Zeng, X.X. Analysis of free amino acids in Chinese teas and flower of tea plant by high performance liquid chromatography combined with solid-phase extraction. Food Chem. 2010, 123, 1259–1266. [Google Scholar] [CrossRef]
- Bai, S.S.; Li, Z.; Zang, X.H.; Wang, C.; Wang, Z. Graphene-based magnetic solid phase extraction dispersive liquid-liquid microextraction combined with gas chromatographic method for determination of five acetanilide herbicides in water and green tea samples. Chin. J. Anal. Chem. 2013, 41, 1177–1182. [Google Scholar] [CrossRef]
- Yılmaz, E.; Soylak, M. Preparation and characterization of magnetic carboxylated nanodiamonds for vortex-assisted magnetic solid-phase extraction of ziram in food and water samples. Talanta 2016, 158, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, M.J.; Wang, S.; Wang, R.Y.; Wu, X.L.; Wen, T.T.; Yuan, L.H.; Dai, P.; Lin, Y.H.; Zhou, X.M. Preparation of imprinted polymers at surface of magnetic nanoparticles for the selective extraction of tadalafil from medicines. ACS App. Mater. Interface 2011, 3, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Chu, J.; Dong, C.K.; Qi, J.Y.; Yuan, Y.X. Synthesis of core-shell magnetic molecular imprinted polymer by the surface RAFT polymerization for the fast and selective removal of endocrine disrupting chemicals from aqueous solutions. Environ. Pollut. 2010, 158, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ding, L.; Long, Y.; Xu, L.G.; Wang, L.B.; Xu, C.L. Preparation and evaluation of superparamagnetic surface molecularly imprinted polymer nanoparticles for selective extraction of bisphenol A in packed food. Anal. Methods 2011, 3, 1737–1744. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Tan, W.; Hu, Y.L.; Li, G.K. Simultaneous determination of trace sterols in complicated biological samples by gas chromatography–mass spectrometry coupled with extraction using β-sitosterol magnetic molecularly imprinted polymer beads. J. Chromatogr. A 2011, 1218, 4275–4283. [Google Scholar] [CrossRef] [PubMed]
- Šafařıiková, M.; Šafařıik, I. Magnetic solid-phase extraction. J. Magn. Magn. Mater. 1999, 194, 108–112. [Google Scholar] [CrossRef]
- Meng, J.; Bu, J.; Deng, C.; Li, G.K. Preparation of polypyrrole-coated magnetic particles for micro solid-phase extraction of phthalates in water by gas chromatography–mass spectrometry analysis. J. Chromatogr. A 2011, 1218, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, G.; Feng, C.; Wang, C.; Wang, Z. Preparation of a graphene-based magnetic nanocomposite for the extraction of carbamate pesticides from environmental water samples. J. Chromatogr. A 2011, 1218, 7936–7942. [Google Scholar] [CrossRef] [PubMed]
- González-Fuenzalida, R.A.; Moliner-Martínez, Y.; Prima-Garcia, H.; Ribera, A.; Campins-Falcó, P.; Zaragozá, R.J. Evaluation of superparamagnetic silica nanoparticles for extraction of triazines in magnetic in-tube solid phase microextraction coupled to capillary liquid chromatography. Nanomaterials 2014, 4, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Moliner-Martinez, Y.; Vitta, Y.; Prima-Garcia, H.; González-Fuenzalida, R.A.; Ribera, A.; Campíns-Falcó, P.; Coronado, E. Silica supported Fe3O4 magnetic nanoparticles for magnetic solid-phase extraction and magnetic in-tube solid-phase microextraction: Application to organophosphorous compounds. Anal. Bioanal. Chem. 2014, 406, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.S.; Miranda, J.M.; Rodriguez, J.A. Magnetic solid phase extraction followed by high-performance liquid chromatography for the determination of sulphonamides in milk samples. Food Chem. 2014, 157, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Arteaga, K.; Rodriguez, J.A.; Barrado, E. Magnetic solids in analytical chemistry: A review. Anal. Chim. Acta 2010, 674, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.S.; Rodriguez, J.A.; Galán-Vidal, C.A.; Cepeda, A.; Miranda, J.M. Magnetic solid phase extraction applied to food analysis. J. Chem. 2015, 8, 1–13. [Google Scholar] [CrossRef]
- Cheng, X.; Yan, H.; Wang, X.; Sun, N.; Qiao, X.Q. Vortex-assisted magnetic dispersive solid-phase microextraction for rapid screening and recognition of dicofol residues in tea products. Food Chem. 2014, 162, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Gray, S. Design of improved deep eutectic solvents using hole theory. Chem. Phys. Chem. 2006, 7, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.C.; Rubio, F.; del Monte, F. Resorcinol-formaldehyde polycondensation in deep eutectic solvents for the preparation of carbons and carbon− carbon nanotube composites. Chem. Mater. 2010, 22, 2711–2719. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Villeneuve, P. Deep eutectic solvents: Synthesis, application, and focus on lipase-catalyzed reactions. Eur. J. Lipid. Sci. Tech. 2013, 115, 379–385. [Google Scholar] [CrossRef]
- Tang, S.; Baker, G.A.; Zhao, H. Ether-and alcohol-functionalized task-specific ionic liquids: Attractive properties and applications. Chem. Soc. Rev. 2012, 41, 4030–4066. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Naka, K.; Chujo, Y. Synthesis of gold nanoparticles modified with ionic liquid based on the imidazolium cation. J. Am. Chem. Soc. 2004, 126, 3026–3027. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Singh, R.K.; Chandra, S. Ionic liquids confined in porous matrices: Physicochemical properties and applications. Prog. Mater. Sci. 2014, 64, 73–120. [Google Scholar] [CrossRef]
- Park, M.J.; Lee, J.K.; Lee, B.S.; Lee, Y.W.; Choi, I.S.; Lee, S.G. Covalent modification of multiwalled carbon nanotubes with imidazolium-based ionic liquids: Effect of anions on solubility. Chem. Mater. 2006, 18, 1546–1551. [Google Scholar] [CrossRef]
- Maran, J.P.; Manikandan, S.; Thirugnanasambandham, K.; Vigna Nivetha, C.; Dinesh, R. Box–Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohyd. Polym. 2013, 92, 604–611. [Google Scholar] [CrossRef] [PubMed]
- De Paula, J.A.M.; Brito, L.F.; Caetano, K.L.F.N.; de Morais Rodrigues, M.L.; Borges, L.L.; da Conceicao, E.C. Ultrasound-assisted extraction of azadirachtin from dried entire fruits of Azadirachta indica A. Juss. (Meliaceae) and its determination by a validated HPLC-PDA method. Talanta 2016, 149, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, G.; Liu, S.; Hu, N.; Geng, D.D.; Chen, G.; Sun, Z.W.; Zhao, X.N.; Xia, L.; You, I.M. Monitoring the contents of six steroidal and phenolic endocrine disrupting chemicals in chicken, fish and aquaculture pond water samples using pre-column derivatization and dispersive liquid–liquid microextraction with the aid of experimental design methodology. Food Chem. 2016, 192, 98–106. [Google Scholar] [PubMed]
- Ghosal, P.S.; Gupta, A.K.; Sulaiman, A. Multivariate optimization of process parameters in the synthesis of calcined Ca‒Al (NO3) LDH for defluoridation using 33 factorial, central composite and Box–Behnken design. J. Environ. Sci. Health. 2016, 51, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Heo, H.J.; Row, K.H. Optimization of crude polysaccharides extraction from Hizikia fusiformis using response surface methodology. Carbohyd. Polym. 2010, 82, 106–110. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Yu, Y.; He, N.Y. Synthesis and characterization of a novel magnetic carrier with its composition of Fe3O4/carbon using hydrothermal reaction. J. Magn. Magn. Mater. 2006, 302, 397–404. [Google Scholar] [CrossRef]
- Kong, X.; Gao, R.; He, X.; Chen, L.X.; Zhang, Y.K. Synthesis and characterization of the core–shell magnetic molecularly imprinted polymers (Fe3O4@MIPs) adsorbents for effective extraction and determination of sulfonamides in the poultry feed. J. Chromatogr. A 2012, 1245, 8–16. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |









| Analyte | Linear Equations | Correlation Coefficient |
|---|---|---|
| Theobromine Theophylline | Y = 9.1044 × 104 + 1.3283 × 104 X Y = −1.0309 × 104 + 6.1173 × 104 X | 0.9993 0.9999 |
| Analyte | Concentration (μg mL−1) | Intra-Day | Inter-Day | ||
|---|---|---|---|---|---|
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | ||
| Theobromine | 10 | 96.74 | 2.06 | 94.34 | 1.31 |
| 50 | 92.68 | 3.32 | 91.89 | 3.17 | |
| 100 | 90.37 | 4.19 | 89.96 | 3.94 | |
| Theophylline | 10 | 95.79 | 2.15 | 93.96 | 2.07 |
| 50 | 91.62 | 4.10 | 92.27 | 3.92 | |
| 100 | 89.13 | 3.36 | 87.72 | 4.03 | |
| Modified Solvents | HBA | HBD | Molar Ratios | Abbreviations |
|---|---|---|---|---|
| DES |  |  | DES-1 | |
 | DES-2 | |||
 | DES-3 | |||
 | 1:2 | DES-4 | ||
 | DES-5 | |||
 | DES-6 | |||
 | DES-7 |
| Modified Solvents | Cation | Anion | Molar Ratios | Abbreviations |
|---|---|---|---|---|
| ILs |  |  | 1:1 | ILs-1 |
 | ILs-2 | |||
 | ILs-3 | |||
 | ILs-4 |
| Variables | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Ratio of methanol (X1) (%) | 0 | 50 | 100 |
| PH of eluent (X2) | 2 | 5 | 8 |
| Volume of eluent (X3) (mL) | 1 | 3.5 | 6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Wang, X.; Row, K.H. Magnetic Solid-phase Extraction with Fe3O4/Molecularly Imprinted Polymers Modified by Deep Eutectic Solvents and Ionic Liquids for the Rapid Purification of Alkaloid Isomers (Theobromine and Theophylline) from Green Tea. Molecules 2017, 22, 1061. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22071061
Li G, Wang X, Row KH. Magnetic Solid-phase Extraction with Fe3O4/Molecularly Imprinted Polymers Modified by Deep Eutectic Solvents and Ionic Liquids for the Rapid Purification of Alkaloid Isomers (Theobromine and Theophylline) from Green Tea. Molecules. 2017; 22(7):1061. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22071061
Chicago/Turabian StyleLi, Guizhen, Xiaoqin Wang, and Kyung Ho Row. 2017. "Magnetic Solid-phase Extraction with Fe3O4/Molecularly Imprinted Polymers Modified by Deep Eutectic Solvents and Ionic Liquids for the Rapid Purification of Alkaloid Isomers (Theobromine and Theophylline) from Green Tea" Molecules 22, no. 7: 1061. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22071061