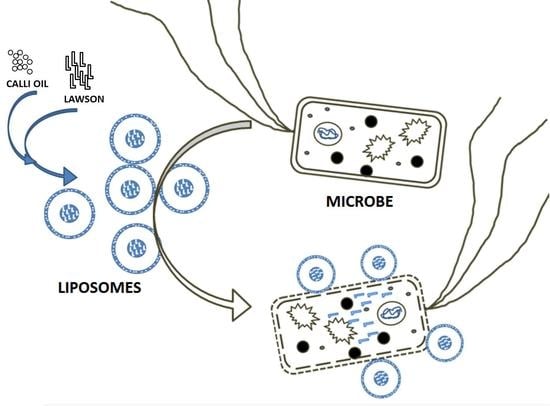
Calli Essential Oils Synergize with Lawsone against Multidrug Resistant Pathogens
Abstract
:1. Introduction
2. Results
2.1. The Anti-Microbial Effect of Lawsone and Calli Oil
2.1.1. Lawsone is a Potential Antimicrobial Candidate
2.1.2. Calligonum Plant Essential Oil (Calli Oil) Showed Potential Antimicrobial Activities
2.2. The Synergistic Effect of both Lawsone and Calli Oil
2.2.1. Calli Oil Augmented the Antimicrobial Activities of Lawsone
2.2.2. Calli Oil Enhanced the Antifungal Activities of Lawsone against Rhizopus Fungus
2.3. The Effect of Liposome Preparation on the Antimicrobial Activity of Lawsone and Calli Oil
2.3.1. Liposome Preparation Enhanced the Antimicrobial Activity of Natural Product Employed
2.3.2. Liposome Preparation Enhanced the Anti-Rhizopus Activity of Natural Product Employed
2.3.3. Liposome Preparation Significantly Reduced the Toxicity of the Natural Product Employed
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Essential Oil Extraction
4.3. Studying the Antimicrobial Activities of Tested Substances
4.4. Stability Testing
4.5. Cytotoxicity Assay
4.5.1. Hemolysis Assay
4.5.2. Mammalian Cell Damage Assay
4.6. Gas Chromatography-Mass Spectrometry (GC-MS)
4.7. Liquid Chromatography-Mass Spectrometry (LC-MS)
4.8. Liposome Preparation and Drug Loading Using Solvent Dispersion Ether Injection (Solvent Vaporization) Method
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patra, A.K. An overview of antimicrobial properties of different classes of phytochemicals. In Dietary Phytochemicals and Microbes; Springer: New York, NY, USA, 2012; pp. 1–32. [Google Scholar]
- Nasiri, H.R.; Madej, M.G.; Panisch, R.; Lafontaine, M.; Bats, J.W.; Lancaster, C.R.D.; Schwalbe, H. Design, synthesis, and biological testing of novel naphthoquinones as substrate-based inhibitors of the quinol/fumarate reductase from Wolinella succinogenes. J. Med. Chem. 2013, 56, 9530–9541. [Google Scholar] [CrossRef] [PubMed]
- Sritrairat, N.; Nukul, N.; Inthasame, P.; Sansuk, A.; Prasirt, J.; Leewatthanakorn, T.; Piamsawad, U.; Dejrudee, A.; Panichayupakaranant, P.; Pangsomboon, K. Antifungal activity of lawsone methyl ether in comparison with chlorhexidine. J. Oral Pathol. Med. 2011, 40, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Rahmoun, N.M.; Boucherit-Otmani, Z.; Boucherit, K.; Benabdallah, M.; Villemin, D.; Choukchou-Braham, N. Antibacterial and antifungal activity of lawsone and novel naphthoquinone derivatives. Med. Mal. Infect. 2012, 42, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.M.G.; Sayeed, S.A.; Ashraf, S.; Naz, S.; Siddiqi, R.; Ali, R.; Mesaik, M.A. A new method for the isolation and purification of lawsone from Lawsonia inermis and its ROS inhibitory activity. Pak. J. Bot. 2013, 45, 1431–1436. [Google Scholar]
- El-Shaer, N.S.; Badr, J.M.; Aboul-Ela, M.A.; Gohar, Y.M. Determination of lawsone in henna powders by high performance thin layer chromatography. J. Sep. Sci. 2007, 30, 3311–3315. [Google Scholar] [CrossRef] [PubMed]
- Sakunphueak, A.; Panichayupakaranant, P. Comparison of antimicrobial activities of naphthoquinones from Impatiens balsamina. Nat. Prod. Res. 2012, 26, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Solanki, B.R.; Gurav, N.C.; Patel, P.H.; Verma, S.S. Method development for lawsone estimation in Trichup herbal hair powder by high-performance thin layer chromatography. J. Adv. Pharm. Technol. Res. 2013, 4, 160–165. [Google Scholar] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food. Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [PubMed]
- Zouari, S.; Dhief, A.; Aschi-Smiti, S. Chemical composition of essential oils of Calligonum comosum cultivated at the south-eastern of Tunisia: A comparative study between flowering and fructification stages. J. Essent. Oil Bear. Plants 2012, 15, 320–327. [Google Scholar] [CrossRef]
- Riadh, H.; Imen, F.; Abdelmajid, Z.; Sinda, F. Detection and extraction of anti-listerial compounds from Calligonum comosum, a medicinal plant from arid regions of Tunisia. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 322–327. [Google Scholar]
- Ebid, A.I. Anti-bacterial activity of folk medicinal plant extracts of Saudi Arabia on isolated bacteria. J. Appl. Life Sci. Int. 2015, 3, 49–54. [Google Scholar] [CrossRef]
- Chouikh, A.; Mekki, M.; Adjal, E. Effects of extraction methods on antibacterial activity of different extracts of Calligonum comosum L’Her. Growing in Sahara Algerian. Int. J. Recent Sci. Res. 2015, 6, 3534–3536. [Google Scholar]
- Aflatuni, A. The Yield and Essential Oil Content of Mint (Mentha ssp.) in Northern Ostrobothnia; Oulu University Press: Oulu, Finland, 2005. [Google Scholar]
- Ullah, I.; Khan, A.L.; Ali, L.; Khan, A.R.; Waqas, M.; Hussain, J.; Lee, I.-J.; Shin, J.-H. Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J. Microbiol. 2015, 53, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Naveed, R.; Hussain, I.; Tawab, A.; Tariq, M.; Rahman, M.; Hameed, S.; Mahmood, M.S.; Siddique, A.B.; Iqbal, M. Antimicrobial activity of the bioactive components of essential oils from Pakistani spices against Salmonella and other multi-drug resistant bacteria. BMC Complement. Altern. Med. 2013, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.; Nosanchuk, J. Melanin and fungi. Curr. Opin. Infect. Dis. 2003, 16, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.; Oluwatuyi, M.; Veitch, N.C.; Gray, A.I. Bacterial resistance modifying agents from Lycopus europaeus. Phytochemistry 2003, 62, 83–87. [Google Scholar] [CrossRef]
- Jones, M.N. The surface properties of phospholipid liposome systems and their characterisation. Adv. Colloid Interface Sci. 1995, 54, 93–128. [Google Scholar] [CrossRef]
- Vemuri, S.; Rhodes, C. Preparation and characterization of liposomes as therapeutic delivery systems: A review. Pharm. Acta Helv. 1995, 70, 95–111. [Google Scholar] [CrossRef]
- Schiffelers, R.; Storm, G.; Bakker-Woudenberg, I. Liposome-encapsulated aminoglycosides in pre-clinical and clinical studies. J. Antimicrob. Chemother. 2001, 48, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Bakker-Woudenberg, I.A.J.M.; Lokerse, A.F.; ten Kate, M.T.; Melissen, P.M.B.; van Vianen, W.; van Etten, E.W.M. Liposomes as carriers of antimicrobial agents or immunomodulatory agents in the treatment of infections. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, S61–S67. [Google Scholar] [CrossRef] [PubMed]
- Sherry, M.; Charcosset, C.; Fessi, H.; Greige-Gerges, H. Essential oils encapsulated in liposomes: A review. J. Liposome Res. 2013, 23, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Wasan, K.M.; Morton, R.E.; Rosenblum, M.G.; Lopez-Berestein, G. Decreased toxicity of liposomal amphotericin B due to association of amphotericin B with high density lipoproteins. J. Pharm. Sci. 1994, 83, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Kokoska, L.; Havlik, J.; Valterova, I.; Sovova, H.; Sajfrtova, M.; Jankovska, I. Comparison of chemical composition and antibacterial activity of Nigella sativa seed essential oils obtained by different extraction methods. J. Food Prot. 2008, 71, 2475–2480. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, W.R.; McAtee, R.K.; Revankar, S.G.; Fothergill, A.W.; McCarthy, D.I.; Rinaldi, M.G.; Patterson, T.F. Comparative evaluation of national committee for clinical laboratory standards broth macrodilution and agar dilution screening methods for testing fluconazole susceptibility of Cryptococcus neoformans. J. Clin. Microbiol. 1998, 36, 1330–1332. [Google Scholar] [PubMed]
- Hasselmann, C. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar]
- Subcommittee of Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 2008, 14, 982–984. [Google Scholar]
- Bokori-Brown, M.; Martin, T.G.; Naylor, C.E.; Basak, A.K.; Titball, R.W.; Savva, C.G. Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein. Nat. Commun. 2016, 7, 11293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, M.; Liu, L.-P.; Deber, C.M. Cationic hydrophobic peptides with antimicrobial activity. Antimicrob. Agents Chemother. 2002, 46, 3585–3590. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Gebremariam, T.; Liu, M.; Chamilos, G.; Kontoyiannis, D.P.; Mink, R.; Kwon-Chung, K.J.; Fu, Y.; Skory, C.D.; Edwards, J.E.; et al. Bacterial endosymbiosis is widely present among Zygomycetes but does not contribute to the pathogenesis of Mucormycosis. J. Infect. Dis. 2008, 198, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Bangham, A.D. Large volume liposomes by an ether vaporization method. BBA Biomembr. 1976, 443, 629–634. [Google Scholar] [CrossRef]
- Schieren, H.; Rudolph, S.; Finkelstein, M.; Coleman, P.; Weissmann, G. Comparison of large unilamellar vesicles prepared by a petroleum ether vaporization method with multilamellar vesicles. BBA Gen. Subj. 1978, 542, 137–153. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, S.S.M.; Alsaadi, A.I.; Youssef, E.G.; Khitrov, G.; Noreddin, A.M.; Husseiny, M.I.; Ibrahim, A.S. Calli Essential Oils Synergize with Lawsone against Multidrug Resistant Pathogens. Molecules 2017, 22, 2223. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22122223
Soliman SSM, Alsaadi AI, Youssef EG, Khitrov G, Noreddin AM, Husseiny MI, Ibrahim AS. Calli Essential Oils Synergize with Lawsone against Multidrug Resistant Pathogens. Molecules. 2017; 22(12):2223. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22122223
Chicago/Turabian StyleSoliman, Sameh S. M., Abrar I. Alsaadi, Eman G. Youssef, Gregory Khitrov, Ayman M. Noreddin, Mohamed I. Husseiny, and Ashraf S. Ibrahim. 2017. "Calli Essential Oils Synergize with Lawsone against Multidrug Resistant Pathogens" Molecules 22, no. 12: 2223. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22122223