Catalytic Enantioselective Addition of Organozirconium Reagents to Aldehydes
Abstract
:1. Introduction
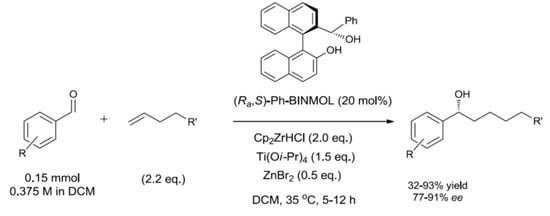
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hanessian, S. Total Synthesis of Natural Products: The Chiron Approach; Pergamon Press: Oxford, UK, 1983; Volume XVIII, 291p, ISBN 0-08-030715-9. [Google Scholar]
- Wang, M.C.; Zhang, Q.-J.; Li, G.-W.; Liu, Z.-K. Highly enantioselective addition of dimethylzinc to arylaldehydes catalyzed by (2S)-1-ferrocenyl-methylaziridin-2-yl(diphenyl)methanol. Tetrahedron Asymmetry 2009, 20, 288–292. [Google Scholar] [CrossRef]
- Sokeirik, Y.S.; Mori, H.; Omote, M.; Sato, K.; Tarui, A.; Kumadaki, I.; Ando, A. Synthesis of a Fluorous Ligand and its Application for Asymmetric Addition of Dimethylzinc to Aldehydes. Org. Lett. 2007, 9, 1927–1929. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, P.G.; Kotrusz, P. Highly Enantioselective Addition of Me2Zn to Aldehydes Catalyzed by ClCr(Salen). J. Am. Chem. Soc. 2006, 128, 4940–4941. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Schneekloth, J., Jr.; Kuramochi, K.; Crews, C.M. Synthetic Studies on Amphidinolide B1. Org. Lett. 2006, 8, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Blay, G.; Fernández, I.; Hernández-Olmos, V.; Marco-Aleixandre, A.; Pedro, J.R. Enantioselective addition of dimethylzinc to aldehydes catalyzed by N-substituted mandelamide-Ti(IV) complexes. Tetrahedron Asymmetry 2005, 16, 1953–1958. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Fukuda, A.; Kimachi, T.; Juichi, M.; Takemoto, Y. Asymmetric synthetic study of macrolactin analogues. Tetrahedron 2005, 61, 2607–2622. [Google Scholar] [CrossRef]
- García-Delgado, N.; Fontes, M.; Pericás, M.A.; Riera, A.; Verdaguer, X. Enantioselective addition of dimethylzinc to aldehydes: Assessment of optimal N,N-substitution for 2-dialkylamino-1,1,2-triphenylethanol ligands. Tetrahedron Asymmetry 2004, 15, 2085–2090. [Google Scholar] [CrossRef]
- Sprout, C.M.; Richmond, M.L.; Seto, C.T. Solid-Phase synthesis of chiral N-acylethylenediamines and their use as ligands for the asymmetric addition of alkylzinc and alkenylzinc reagents to aldehydes. J. Org. Chem. 2004, 69, 6666–6673. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, P.G.; Locatelli, M. Catalytic Enantioselective Addition of Me2Zn to Aromatic Aldehydes Promoted by New Modular Thiophene-Oxazoline Ligands. Lett. Org. Chem. 2004, 1, 208–211. [Google Scholar] [CrossRef]
- Jones, G.B.; Huber, R.S.; Chapman, B.J. Catalytic enantioselective synthesis of macrolides via asymmetric alkylation. Tetrahedron: Asymmetry 1997, 8, 1797–1809. [Google Scholar] [CrossRef]
- Kitamura, M.; Suga, S.; Kawai, K.; Noyori, R. Catalytic asymmetric induction. Highly enantioselective addition of dialkylzincs to aldehydes. J. Am. Chem. Soc. 1986, 108, 6071–6072. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.M.; Singaram, B. Asymmetric addition of diorganozinc reagents to aldehydes and ketones. Org. Prep. Proced. Int. 2011, 43, 139–208. [Google Scholar] [CrossRef]
- Ramón, D.J.; Yus, M. In the arena of enantioselective synthesis, titanium complexes wear the laurel wreath. Chem. Rev. 2006, 106, 2126–2208. [Google Scholar] [CrossRef] [PubMed]
- Yus, M.; Ramón, D.J. Enantioselective addition of organozinc reagents to carbonyl compounds. Pure Appl. Chem. 2005, 77, 2111–2119. [Google Scholar] [CrossRef]
- Yus, M.; Ramón, D.J. Recent developments in the enantioselective 1,2-addition of organometallic reagents to carbonylic compounds. Recent Res. Dev. Org. Chem. 2002, 6, 297–378. [Google Scholar]
- Pu, L.; Yu, H.-B. Catalytic asymmetric organozinc additions to carbonyl compounds. Chem. Rev. 2001, 101, 757–824. [Google Scholar] [CrossRef] [PubMed]
- Mata, Y.; Dièguez, M.; Pàmies, O.; Woodward, S. Screening of a Modular Sugar-Based Phosphite Ligand Library in the Asymmetric Nickel-Catalyzed Trialkylaluminum Addition to Aldehydes. J. Org. Chem. 2006, 71, 8159–8165. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Prieto, O.; Goldsmith, P.; Woodward, S. Remarkably Stable (Me3Al)2⋅DABCO and Stereoselective Nickel-Catalyzed AlR3 (R = Me, Et) Additions to Aldehydes. Angew. Chem. Int. Ed. 2005, 44, 2232–2234. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mateos, E.; Maciá, B.; Yus, M. Catalytic enantioselective addition of organoaluminum reagents to aldehydes. Tetrahedron Asymmetry 2012, 23, 789–794. [Google Scholar] [CrossRef]
- Fernández-Mateos, E.; Maciá, B.; Yus, M. Catalytic Enantioselective Addition of Alkyl Grignard Reagents to Aliphatic Aldehydes. Adv. Synth. Catal. 2013, 355, 1249–1254. [Google Scholar] [CrossRef]
- Zheng, L.-S.; Jiang, K.-Z.; Deng, Y.; Bai, X.-F.; Gao, G.; Gu, F.-L.; Xu, L.-W. Synthesis of Ar-BINMOL Ligands by [1,2]-Wittig Rearrangement to Probe Their Catalytic Activity in 1,2-Addition Reactions of Aldehydes with Grignard Reagents. Eur. J. Org. Chem. 2013, 4, 748–755. [Google Scholar] [CrossRef]
- Fernández-Mateos, E.; Maciá, B.; Ramón, D.J.; Yus, M. Catalytic Enantioselective Addition of MeMgBr and Other Grignard Reagents to Aldehydes. Eur. J. Org. Chem. 2011, 2011, 6851–6855. [Google Scholar] [CrossRef]
- Itakura, D.; Harada, T. Catalytic Enantioselective Arylation of Aldehydes by Using Functionalized Grignard Reagents Generated from Aryl Bromides. Synlett 2011, 2011, 2875–2879. [Google Scholar] [CrossRef]
- Liu, Y.; Da, C.-S.; Yu, S.-L.; Yin, X.-G.; Wang, J.-R.; Fan, X.-Y.; Li, W.-P.; Wang, R. Catalytic Highly Enantioselective Alkylation of Aldehydes with Deactivated Grignard Reagents and Synthesis of Bioactive Intermediate Secondary Arylpropanols. J. Org. Chem. 2010, 75, 6869–6878. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-Y.; Yang, Y.-X.; Zhuo, F.-F.; Yu, S.-L.; Li, X.; Guo, Q.-P.; Du, Z.-X.; Da, C.-S. AlCl3 and BDMAEE: A Pair of Potent Reactive Regulators of Aryl Grignard Reagents and Highly Catalytic Asymmetric Arylation of Aldehydes. Chem. Eur. J. 2010, 16, 7988–7991. [Google Scholar] [CrossRef] [PubMed]
- Da, C.-S.; Wang, J.-R.; Yin, X.-G.; Fan, X.-Y.; Liu, Y.; Yu, S.-L. Highly Catalytic Asymmetric Addition of Deactivated Alkyl Grignard Reagents to Aldehydes. Org. Lett. 2009, 11, 5578–5581. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, Y.; Harada, T. Catalytic Asymmetric Alkylation of Aldehydes with Grignard Reagents. Angew. Chem. Int. Ed. 2008, 47, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, Y.; Harada, T. Catalytic Asymmetric Aryl Transfer Reactions to Aldehydes with Grignard Reagents as the Aryl Source. Chem. Eur. J. 2008, 14, 10560–10563. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mateos, E.; Maciá, B.; Yus, M. Catalytic Enantioselective Addition of Aryl Grignard Reagents to Ketones. Eur. J. Org. Chem. 2014, 2014, 6519–6526. [Google Scholar] [CrossRef]
- Pellissier, H. Enantioselective Titanium-promoted 1,2-additions of Carbon Nucleophiles to Carbonyl Compounds. Tetrahedron 2015, 71, 2487–2524. [Google Scholar] [CrossRef]
- Madduri, A.V.R.; Harutyunyan, S.R.; Minnaard, A.J. Asymmetric Copper-Catalyzed Addition of Grignard Reagents to Aryl Alkyl Ketones. Angew. Chem. Int. Ed. 2012, 51, 3164–3167. [Google Scholar] [CrossRef] [PubMed]
- Madduri, A.V.R.; Minnaard, A.J.; Harutyunyan, S.R. Copper(I) catalyzed asymmetric 1,2-addition of Grignard Reagents to α-methyl Substituted α,β-unsaturated Ketones. Chem. Commun. 2012, 48, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Collados, J.F.; Solà, R.; Harutyunyan, S.R.; Maciá, B. Catalytic Synthesis of Enantiopure Chiral Alcohols via Addition of Grignard Reagents to Carbonyl Compounds. ACS Catal. 2016, 6, 1952–1970. [Google Scholar] [CrossRef]
- Bieszczad, B.; Gilheany, D.G. Asymmetric Grignard Synthesis of Tertiary Alcohols through Rational Ligand Design. Angew. Chem. Int. Ed. 2017, 56, 4272–4276. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mateos, E.; Maciá, B.; Yus, M. Catalytic Asymmetric Addition of Alkyllithium Reagents to Aromatic Aldehydes. Eur. J. Org. Chem. 2012, 2012, 3732–3736. [Google Scholar] [CrossRef]
- Veguillas, M.; Solà, R.; Shaw, L.; Maciá, B. Catalytic Asymmetric Addition of Organolithium Reagents to Aldehydes. Eur. J. Org. Chem. 2016, 9, 1788–1794. [Google Scholar] [CrossRef]
- Zong, H.; Huang, H.; Song, L. Catalytic Aasymmetric Addition of Aldehydes Using Organolithium Reagents in the Presence of Commercial Available Chiral Diol Ligands. Tetrahedron Asymmetry 2016, 27, 1069–1074. [Google Scholar] [CrossRef]
- Howell, G.P. Asymmetric and Diastereoselective Conjugate Addition Reactions: C–C Bond Formation at Large Scale. Org. Process Res. Dev. 2012, 16, 1258–1272. [Google Scholar] [CrossRef]
- Harutyunyan, S.R.; den Hartog, T.; Geurts, K.; Minnaard, A.J.; Feringa, B.L. Catalytic Asymmetric Conjugate Addition and Allylic Alkylation with Grignard Reagents. Chem. Rev. 2008, 108, 2824–2852. [Google Scholar] [CrossRef] [PubMed]
- Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F.F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V.A. Highly Functionalized Organomagnesium Reagents Prepared through Halogen–Metal Exchange. Angew. Chem. Int. Ed. 2003, 42, 4302–4320. [Google Scholar] [CrossRef] [PubMed]
- Negishi, E.I.; Huo, S.; Takahashi, T.; Li, Y.; Dixon, S.; Whitby, R.J.; Hanzawa, Y. Titanium and Zirconium in Organic Synthesis; Marek, I., Ed.; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Buchwald, S.L.; Nielsen, R.B. Group 4 Metal Complexes of Benzynes, Cycloalkynes, Acyclic Alkynes, and Alkenes. Chem. Rev. 1988, 88, 1047–1058. [Google Scholar] [CrossRef]
- Bauer, T. Enantioselective Dialkylzinc-mediated Alkynylation, Arylation and Alkenylation of Carbonyl Groups. Coord. Chem. Rev. 2015, 299, 83–150. [Google Scholar] [CrossRef]
- Negishi, E.-I. Organozirconium Chemistry. In Organometallics in Synthesis: A Manual, 2nd ed.; Schlosser, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Negishi, E.-I.; Takahashi, T. Organozirconium Compounds in Organic Synthesis. Synthesis 1988, 1, 1–19. [Google Scholar] [CrossRef]
- Hart, D.W.; Schwartz, J. Hydrozirconation. Organic Synthesis via Organozirconium Intermediates. Synthesis and Rearrangement of Alkylzirconium(IV) Complexes and Their Reaction with Electrophiles. J. Am. Chem. Soc. 1974, 96, 8115–8116. [Google Scholar] [CrossRef]
- Negishi, E.-I. A Quarter of a Century of Explorations in Organozirconium Chemistry. Dalton Trans. 2005, 5, 827–848. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Palumbo, G.; Caputo, R. Organotitanium and Organozirconium Reagents in Comprehensive Organic Synthesis, Additions to C–X π-Bonds; Trost, B.M., Ed.; Elsevier Ltd.: Oxford, UK, 1991; Volume 1. [Google Scholar]
- Carr, D.B.; Schwartz, J. Preparation of organoaluminum compounds by hydrozirconation-transmetalation. J. Am. Chem. Soc. 1979, 101, 3521–3531. [Google Scholar] [CrossRef]
- Venanzi, L.M.; Lehmann, R.; Keil, R.; Lipshutz, B.H. Copper-catalyzed allylic alkylations of alkylzirconium intermediates. Tetrahedron Lett. 1992, 33, 5857–5860. [Google Scholar] [CrossRef]
- Babiak, K.A.; Behling, J.R.; Dygos, J.H.; McLaughlin, K.T.; Ng, J.S.; Kalish, V.J.; Kramer, S.W.; Shone, R.L. One-pot synthesis of protected prostaglandins from alkynes and cyclopentenones. In situ generation of higher order cyanocuprates derived from alkenylzirconium intermediates. J. Am. Chem. Soc. 1990, 112, 7441–7442. [Google Scholar] [CrossRef]
- Deloux, L.; Skrzypczak-Jankun, E.; Cheesman, B.V.; Srebnik, M.; Sabat, M. First Example of Stable 1,1-Bimetalloalkenes of Boron and Zirconium: Synthesis, Reactivity, X-ray Analysis, and NMR Studies. J. Am. Chem. Soc. 1994, 116, 10302–10303. [Google Scholar] [CrossRef]
- Sun, A.; Huang, X. Stereoselective Preparation of α-Heteroatom Substituted α,β-Unsaturated Ketones. Synthesis 2000, 6, 775–777. [Google Scholar] [CrossRef]
- Wipf, P.; Xu, W. Transmetalation Reactions of Organozirconocenes: A General, Selective, and Facile Synthesis of Ketones from Acid Chlorides. Synlett 1992, 9, 718–721. [Google Scholar] [CrossRef]
- Wipf, P.; Kendall, C. Novel Applications of Alkenyl Zirconocenes. Chem. Eur. J. 2002, 8, 1778–1784. [Google Scholar] [CrossRef]
- Negishi, E.; Okukado, N.; King, A.O.; van Horn, D.E.; Spiegel, B.I. Selective carbon-carbon bond formation via transition metal catalysts. 9. Double metal catalysis in the cross-coupling reaction and its application to the stereo- and regioselective synthesis of trisubstituted olefins. J. Am. Chem. Soc. 1978, 100, 2254–2256. [Google Scholar] [CrossRef]
- Thompson, C.F.; Jamison, T.F.; Jacobsen, E.N. FR901464: Total Synthesis, Proof of Structure, and Evaluation of Synthetic Analogues. J. Am. Chem. Soc. 2001, 123, 9974–9983. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Novel Lewis acid catalysis in organic synthesis. Pure Appl. Chem. 1994, 66, 1557–1564. [Google Scholar] [CrossRef]
- Suzuki, K.; Hasegawa, T.; Imai, T.; Maeta, H.; Ohba, S. AgAsF6 as Safe Alternative to AgClO4 for Generating Cationic Zirconocene Species: Utilities in Lewis Acid-Promoted Selective C–C Bond Forming Reactions. Tetrahedron 1995, 51, 4483–4494. [Google Scholar] [CrossRef]
- Maeta, H.; Hashimoto, T.; Hasegawa, T.; Suzuki, K. Grignard-type Addition of Alkenyl- and Alkylzirconocene Chloride to Aldehyde: Remarkable Catalytic Acceleration Effect of AgClO4. Tetrahedron Lett. 1992, 33, 5965–5968. [Google Scholar] [CrossRef]
- Zheng, B.; Srebnik, M. 1,2-Addition of Alkyl- and Alkenylzirconocene Chlorides to Aldehydes Accelerated by Catalytic Amounts of ZnBr2 as a Method of Synthesizing Secondary Alcohols, Secondary Allylic Alcohols, and in-Situ Oppenauer-Type Oxidation of the Alcohols to Ketones. J. Org. Chem. 1995, 60, 3278–3279. [Google Scholar] [CrossRef]
- Wipf, P.; Xu, W. Preparation of allylic alcohols by alkene transfer from zirconium to zinc. Tetrahedron Lett. 1994, 35, 5197–5200. [Google Scholar] [CrossRef]
- Wipf, P.; Xu, W. Allylic Alcohols by Alkene Transfer from Zirconium to Zinc: 1-[(tert-butyldiphenylsilyl)oxy]-dec-3-en-5-ol. Org. Synth. 1997, 74, 205. [Google Scholar]
- Yamamoto, Y.; Maruyama, K. Crotylzirconium derivatives as a new reagent for the threo selective synthesis of β-methylhomoallyl alcohols. Tetrahedron Lett. 1981, 22, 2895–2898. [Google Scholar] [CrossRef]
- Mashima, K.; Yasuda, H.; Asami, K.; Nakamura, A. Structures of Mono- and Bis(2-butenyl)zirconium complexes in solution and threo selective insertion reaction of aliphatic aldehydes. Chem. Lett. 1983, 12, 219–222. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Saito, Y.; Maruyama, K. The influence of (organo)metallics “metal-tuning” on stereo- and regio-chemical convergence in reactions of allylic carbanions with aldehydes. J. Organomet. Chem. 1985, 292, 311–318. [Google Scholar] [CrossRef]
- Fan, G.; Xie, X.; Liu, Y.; Li, Y. Unusual Regioselectivity in the Aldehyde Addition Reactions of Allenyl/Propargyl Zirconium Complexes Derived from γ-(2-Pyridyl)propargyl Ethers: Synthesis of Multisubstituted α-Hydroxyallenes. Organometallics 2013, 32, 1636–1642. [Google Scholar] [CrossRef]
- Weidman, B.; Maycock, C.D.; Seebach, D. Alkyl-, Aryl-, Vinyl-, and Heterosubstituted Organozirconium Compounds. -Selective nucleophiles of low basicity. Preliminary communication. Helv. Chim. Acta 1981, 64, 1552–1557. [Google Scholar] [CrossRef]
- Weidman, B.; Seebach, D. Organometallic Compounds of Titanium and Zirconium as Selective Nucleophilic Reagents in Organic Synthesis. Angew. Chem. Int. Ed. 1983, 22, 31–45. [Google Scholar] [CrossRef]
- Loots, M.J.; Schwartz, J. Nickel-catalyzed conjugate addition of zirconium alkenyls to α,β-Unsaturated Ketones. J. Am. Chem. Soc. 1977, 99, 8045–8046. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Ellsworth, E.L. Hydrozirconation-transmetalation. A mild, direct route to higher order vinylic cuprates from monosubstituted acetylenes. J. Am. Chem. Soc. 1990, 112, 7440–7441. [Google Scholar] [CrossRef]
- Wipf, P.; Smitrovich, J.H. Transmetalation reactions of alkylzirconocenes: Copper-catalyzed conjugate addition to enones. J. Org. Chem. 1991, 56, 6494–6496. [Google Scholar] [CrossRef]
- Wipf, P.; Xu, W.J.; Smitrovich, J.H.; Lehmann, R.; Venanzi, L.M. Copper-catalyzed conjugate additions of organozirconocenes. Synthetic and mechanistic studies. Tetrahedron 1994, 50, 1935–1954. [Google Scholar] [CrossRef]
- Wipf, P.; Xu, W. Organozirconocenes in organic synthesis: Tandem epoxide rearrangement-carbonyl addition. J. Org. Chem. 1993, 58, 825–826. [Google Scholar] [CrossRef]
- Negishi, E.; Swanson, D.R.; Miller, S.R. One-pot conversion of alkynes and alkenes into one-carbon homologated aldehydes via hydrozirconation-isocyanide insertion-hydrolysis. Tetrahedron Lett. 1988, 29, 1631–1634. [Google Scholar] [CrossRef]
- Wipf, P.; Stephenson, C.R. Dimethylzinc-Mediated Addition of Alkenylzirconocenes to α-Keto and α-Imino Esters. Org. Lett. 2003, 5, 2449–2452. [Google Scholar] [CrossRef] [PubMed]
- Wipf, P.; Takahashi, H. Copper(I)-catalysed asymmetric conjugate addition of organozirconocenes to N-acyl oxazolidinones. Chem. Commun. 1996, 23, 2675–2676. [Google Scholar] [CrossRef]
- Chavez, D.E.; Jacobsen, E.N. Total Synthesis of Fostriecin (CI-920). Angew. Chem. Int. Ed. 2001, 40, 3667–3670. [Google Scholar] [CrossRef]
- Lou, S.; Fu, G.C. Enantioselective Alkenylation via Nickel-Catalyzed Cross-Coupling with Organozirconium Reagents. J. Am. Chem. Soc. 2010, 132, 5010–5011. [Google Scholar] [CrossRef] [PubMed]
- Westmeier, J.; Pfaff, C.; Siewert, J.; von Zezschwitz, P. First Tandem Asymmetric Conjugate Addition of Alkenyl Nucleophiles and Silyl Trapping of the Intermediate Enolates. Adv. Synth. Catal. 2013, 355, 2651–2658. [Google Scholar] [CrossRef]
- Roth, P.M.C.; Fletcher, S.P. Enantioselective Copper(I)-Phosphoramidite Catalyzed Addition of Alkylzirconium Species to Acyclic Enones. Org. Lett. 2015, 17, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Sidera, M.; Roth, P.M.C.; Maksymowicz, R.M.; Fletcher, S.P. Formation of Quaternary Centers by Copper-Catalyzed Asymmetric Conjugate Addition of Alkylzirconium Reagents. Angew. Chem. Int. Ed. 2013, 52, 7995–7999. [Google Scholar] [CrossRef] [PubMed]
- Roth, P.M.C.; Sidera, M.; Maksymowicz, R.M.; Fletcher, S.P. Copper-catalyzed asymmetric conjugate addition of alkylzirconium reagents to cyclic enones to form quaternary centers. Nat. Protoc. 2013, 9, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Mäsing, F.; Fletcher, S.P. Asymmetric Conjugate Addition of Alkylzirconocenes to Cyclopent-4-ene-1,3-dione Monoacetals. Synthesis 2015, 47, 2217–2222. [Google Scholar]
- Maksymowicz, R.M.; Roth, P.M.C.; Fletcher, S.P. Catalytic asymmetric carbon–carbon bond formation using alkenes as alkylmetal equivalents. Nat. Chem. 2012, 4, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Maksymowicz, R.M.; Sidera, M.; Roth, P.M.C.; Fletcher, S.P. A Convenient Catalytic Asymmetric Conjugate Addition Reaction to Enones Using Alkylzirconium Reagents. Synthesis 2013, 45, 2662–2668. [Google Scholar] [CrossRef]
- Maciver, E.E.; Maksymowicz, R.M.; Wilkinson, N.; Roth, P.M.C.; Fletcher, S.P. Asymmetric Conjugate Addition of Alkylzirconium Reagents to α,β-Unsaturated Lactones. Org. Lett. 2014, 16, 3288–3291. [Google Scholar] [CrossRef] [PubMed]
- Caprioglio, D.; Fletcher, S.P. An alternative synthesis of the breast cancer drug fulvestrant (Faslodex®): Catalyst control over C–C bond formation. Chem. Commun. 2015, 51, 14866–14868. [Google Scholar] [CrossRef] [PubMed]
- Maksymowicz, R.M.; Roth, P.M.C.; Thompson, A.L.; Fletcher, S.P. Hydrometallation-asymmetric conjugate addition: Application to complex molecule synthesis. Chem. Commun. 2013, 49, 4211–4213. [Google Scholar] [CrossRef] [PubMed]
- Mola, L.; Sidera, M.; Fletcher, S.P. Asymmetric Remote C–H Functionalization: Use of Internal Olefins in Tandem Hydrometallation–Isomerization–Asymmetric Conjugate Addition Sequences. Aust. J. Chem. 2015, 68, 401–403. [Google Scholar] [CrossRef]
- Garrec, K.; Fletcher, S.P. Cp2ZrMeCl: A Reagent for Asymmetric Methyl Addition. Org. Lett. 2016, 18, 3814–3817. [Google Scholar] [CrossRef] [PubMed]
- Wipf, P.; Jayasuriya, N.; Ribe, S. On the role of chiral catalysts in the alkenyl zirconocene/zinc addition to aldehydes: A study of ligand loading and asymmetric amplification. Chirality 2003, 15, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Wipf, P.; Ribe, S. Zirconocene−Zinc Transmetalation and in-Situ Catalytic Asymmetric Addition to Aldehydes. J. Org. Chem. 1998, 63, 6454–6455. [Google Scholar] [CrossRef]
- Li, H.; Walsh, P.J. Catalytic Asymmetric Vinylation and Dienylation of Ketones. J. Am. Chem. Soc. 2005, 127, 8355–8361. [Google Scholar] [CrossRef] [PubMed]
- Pu, L. Asymmetric Functional Organozinc Additions to Aldehydes Catalyzed by 1,1′-Bi-2-naphthols (BINOLs). Acc. Chem. Res. 2014, 47, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Labinger, J.A. Hydrozirconation: A New Transition Metal Reagent for Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1976, 15, 333–340. [Google Scholar] [CrossRef]
- Buchwald, S.L.; LaMaire, S.J.; Nielsen, R.B. Schwartz’s Reagent. Org. Synth. 1993, 71, 77–82. [Google Scholar]
- Wipf, P.; Jahn, H. Synthetic applications of organochlorozirconocene complexes. Tetrahedron 1996, 52, 12853–12910. [Google Scholar] [CrossRef]
- Patai, S. The Chemistry of the Double Bonded Functional Groups; Wiley: Chichester, UK, 1997. [Google Scholar]
- Uhlig, E.; Bürglen, B.; Krüger, C.; Betz, P. Die reaktivitätsabstufung von trimethylsiloxygruppen gegenüber dem Hydrozirconierungsreagenz Cp2Zr(H)Cl. J. Organomet. Chem. 1990, 382, 77–88. [Google Scholar] [CrossRef]
- Gao, G.; Gu, F.-L.; Jiang, J.-X.; Jiang, K.; Sheng, C.-Q.; Lai, G.-Q.; Xu, L.-W. Neighboring Lithium-Assisted [1,2]-Wittig Rearrangement: Practical Access to Diarylmethanol-Based 1,4-Diols and Optically Active BINOL Derivatives with Axial and sp3-Central Chirality. Chem. Eur. J. 2011, 17, 2698–2703. [Google Scholar] [CrossRef] [PubMed]
- Kiyooka, S.-I.; Tsutsui, T.; Kira, T. Complete asymmetric induction in [1,2]-wittig rearrangement of a system involving a binaphthol moiety. Tetrahedron Lett. 1996, 37, 8903–8904. [Google Scholar] [CrossRef]
- Gao, G.; Bai, X.-F.; Yang, H.-M.; Jiang, J.-X.; Lai, G.-Q.; Xu, L.W. Ar-BINMOLs with Axial and sp3 Central Chirality–Characterization, Chiroptical Properties, and Application in Asymmetric Catalysis. Eur. J. Org. Chem. 2011, 2011, 5039–5046. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, L.-W. Development of Ar-BINMOL-Derived Atropisomeric Ligands with Matched Axial and sp3 Central Chirality for Catalytic Asymmetric Transformations. Chem. Rec. 2015, 15, 925–948. [Google Scholar] [CrossRef] [PubMed]
- Panek, J.S.; Hu, T. Asymmetric Synthesis of (2S,3S,8S,9S)-N-Boc ADDA: Application of a Palladium(0)-Catalyzed Cross-Coupling Reaction of Trisubstituted Olefins. J. Org. Chem. 1997, 62, 4914–4915. [Google Scholar] [CrossRef]
- Ho, T.; Panek, J.S. Total Synthesis of (−)-Motuporin. J. Org. Chem. 1999, 64, 3000–3001. [Google Scholar]
- Drouet, K.E.; Theodorakis, E.A. Enantioselective Total Synthesis of Reveromycin B. J. Am. Chem. Soc. 1999, 121, 456–457. [Google Scholar] [CrossRef]
- Muller, B.; Ruf, M.; Vahrenkamp, H. On the Nature of Zinc Chloride–Aldehyde Interactions. Angew. Chem. Int. Ed. Engl. 1994, 33, 2089–2090. [Google Scholar] [CrossRef]
- Jordan, R.F. Chemistry of Cationic Dicyclopentadienyl Group 4 Metal-Alky I Complexes. Adv. Organomet. Chem. 1991, 32, 325–387. [Google Scholar]
- Veguillas, M.; Solà, R.; Fernández-Ibáñez, M.A.; Maciá, B. Catalytic enantioselective addition of methyltriisopropoxititanium to aldehydes. Tetrahedron Asymmetry 2016, 27, 643–648. [Google Scholar] [CrossRef]
- Boivin, T.L.B. Synthetic routes to tetrahydrofuran, tetrahydropyran, and spiroketal units of polyether antibiotics and a survey of spiroketals of other natural products. Tetrahedron 1987, 43, 3309–3362. [Google Scholar] [CrossRef]
- Cradillo, G.; Orena, M. Stereocontrolled cyclofunctionalizations of double bonds through heterocyclic intermediates. Tetrahedron 1990, 46, 3321–3408. [Google Scholar] [CrossRef]
- Kotsuki, H. Bicyclic Ketals: Versatile Intermediates for the Stereocontrolled Construction of Cyclic Ether Derivatives. Synlett 1992, 1992, 97–106. [Google Scholar] [CrossRef]
- Bartlett, P.A. Stereocontrol in the synthesis of acyclic systems: Applications to natural product synthesis. Tetrahedron 1980, 36, 2–72. [Google Scholar] [CrossRef]
- Elliott, M.C.; Williams, E. Saturated oxygen heterocycles. J. Chem. Soc. Perkin Trans. 2001, 1, 2303–2340. [Google Scholar] [CrossRef]
- Narula, A.P.S. The Search for New Fragrance Ingredients for Functional Perfumery. Chem. Biodivers. 2004, 1, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Loman, J.J.; Carnaghan, E.R.; Hamlin, T.A.; Ovian, J.M.; Kelly, C.B.; Mercadante, M.A.; Leadbeater, N.E. A combined computational and experimental investigation of the oxidative ring-opening of cyclic ethers by oxoammonium cations. Org. Biomol. Chem. 2016, 14, 3883–3888. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.H.; Pellon, P. Chiral organosilicon compounds in synthesis. Highly enantioselective synthesis of arylcarbinols. J. Am. Chem. Soc. 1989, 111, 8737–8738. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound 3 are available from the authors. |



| Entry | Cp2ZrHCl (eq.) | 1-hexene (eq.) | Ti(OiPr)4 (eq.) | ZnBr2 (eq.) | Conv. (%) b | Undesired Phenylmethanol (%) b | ee (%) c |
|---|---|---|---|---|---|---|---|
| 1 d | 2 | 2 | - | - | 13 | 0 | 0 |
| 2 d | 2 | 2 | - | - e | 19 | 0 | 0 |
| 3 | 2 | 2 | - | - e | 44 | 0. | |
| 4 | 1.2 | 1.4 | 1.5 | 0.5 | n.d. (83) f | 10 | 80 |
| 5 g | 1.2 | 1.4 | 1.5 | 0.5 | 1 | n.d. | 0 |
| 6 | 1.2 | 1.4 | - h | 0.5 | 22 | 78 | 0 |
| 7 | 1.2 | 1.4 | 1.0 | 0.5 | 43 | 57 | 35 |
| 8 | 1.2 | 1.4 | 2.0 | 0.5 | 5 | 89 | 62 |
| 9 | 1.2 | 1.4 | 1.5 | 0.2 | 18 | 73 | 80 |
| 10 | 1.2 | 1.4 | 1.5 | 0.7 | 20 | 74 | 66 |
| 11 i | 1.2 | 1.4 | 1.5 | 0.5 | 6 | 67 | 35 |
| 12 j | 1.2 | 1.4 | 1.5 | 0.5 | 51 | 36 | 82 |
| 13 k | 1.2 | 1.4 | 1.5 | 0.5 | 9 | 83 | 56 |
| 14 | 1.0 | 1.2 | 1.5 | 0.5 | 11 | 59 | 90 |
| 15 j | 2.0 | 2.2 | 1.5 | 0.5 | 99 (87) f | 5 | 93 (R) l |

| Entry | Product | Conv. (%) b | Undesired Arylmethanol (%) b | Yield (%) c | ee (%) d |
|---|---|---|---|---|---|
| 1 |  | 94 | 6 | 74 | 91 (R) |
| 2 |  | 77 | 15 e | 54 | 89 (R) |
| 3 |  | 54 | 28 f | 49 | 76 (R) |
| 4 |  | 87 | 10 | 56 | 91 (R) |
| 5 g |  | 92 | 6 | 59 | 90 (R) |
| 6 |  | 76 | 4 h | 32 | 94 (R) |
| 7 f |  | 81 | 19 | 58 | 87 (R) |
| 8 |  | 69 | 28 | 55 | 87 i (R) |
| Entry | Product | Conv. (%) b | Yield (%) c | ee (%) d |
|---|---|---|---|---|
| 1 e |  | >99 | 93 | 77 (R) |
| 2 |  | n.d. | 42 | 88 (R) |
| 3 |  | 75 (10) f | 40 | 85 g (R) |
| 4 |  | 67 | 41 | 74 (R) |
| 5 |  | 61 | 31 | 81 (R) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solà, R.; Veguillas, M.; González-Soria, M.J.; Carter, N.; Fernández-Ibáñez, M.A.; Maciá, B. Catalytic Enantioselective Addition of Organozirconium Reagents to Aldehydes. Molecules 2018, 23, 961. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23040961
Solà R, Veguillas M, González-Soria MJ, Carter N, Fernández-Ibáñez MA, Maciá B. Catalytic Enantioselective Addition of Organozirconium Reagents to Aldehydes. Molecules. 2018; 23(4):961. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23040961
Chicago/Turabian StyleSolà, Ricard, Marcos Veguillas, María José González-Soria, Nicholas Carter, M. Angeles Fernández-Ibáñez, and Beatriz Maciá. 2018. "Catalytic Enantioselective Addition of Organozirconium Reagents to Aldehydes" Molecules 23, no. 4: 961. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23040961