A Comprehensive Study on the Dye Adsorption Behavior of Polyoxometalate-Complex Nano-Hybrids Containing Classic β-Octamolybdate and Biimidazole Units
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Strategy
2.2. Description of Crystal Structures of Complexes
2.3. PXRD Patterns and Thermal Analyses
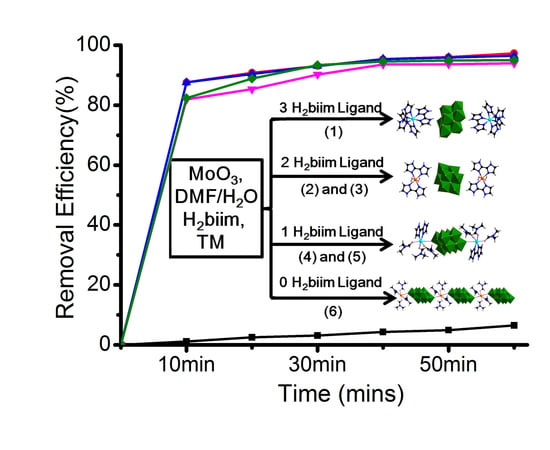
2.4. Adsorption of Organic Dyes
3. Materials and Methods
3.1. Synthesis of 2,2′-Biimidazole Ligand
3.2. Synthesis of [Ni(H2biim)3]2[Mo8O26]•8DMF (1)
3.3. Synthesis of (DMA)2[Ni(H2biim)2(H2O)2][Mo8O26]•4DMF (2)
3.4. Synthesis of (DMA)2[Co(H2biim)2(H2O)2][Mo8O26]•4DMF (3)
3.5. Synthesis of [Zn(H2biim)(DMF)3]2[Mo8O26]•2DMF (4)
3.6. Synthesis of [Cu(H2biim)(DMF)3]2[Mo8O26]•2DMF (5) and (DMA)2[Cu(DMF)4][Mo8O26]•2DMF (6)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.S.; Liang, J.; Li, L.; Lin, Z.J.; Bag, P.P.; Gao, S.Y.; Huang, Y.B.; Cao, R. An anion metal-organic framework with Lewis Basic sites-rich toward charge-exclusive cationic dyes separation and size-selective catalytic reaction. Inorg. Chem. 2016, 55, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.; Tian, J.Y.; Wang, Z.W.; Liu, C.S.; Chen, M.; Du, M. An anionic Na(i)-organic framework platform: separation of organic dyes and post-modification for highly sensitive detection of picric acid. Chem.Commun. 2017, 53, 10668–10671. [Google Scholar] [CrossRef] [PubMed]
- He, Y.C.; Yang, J.; Kan, W.Q.; Zhang, H.M.; Liu, Y.Y.; Ma, J.F. A new microporous anionic metal–organic framework as a platform for highly selective adsorption and separation of organic dyes. J. Mater. Chem. A. 2015, 3, 1675–1681. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, Y.L.; Zhang, L.; Sun, D.; Fang, J.; Zhu, S. Two nanocage anionic metal-organic frameworks with rht topology and {[M(H2O)6]6}(12+) charge aggregation for rapid and selective adsorption of cationic dyes. Chem.Commun. 2014, 50, 14674–14677. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.K.; Yi, F.Y.; Fang, Y.; Xiao, X.W.; Wang, S.C.; Pan, L.Q.; Zhu, S.R.; Tao, K.; Han, L. An ultrastable metal–organic framework with open coordinated sites realizing selective separation toward cationic dyes in aqueous solution. Cryst. Growth. Des. 2017, 17, 5458–5464. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, X.; Qian, X.; Dong, M. Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes. J. Colloid. Interf. Sci. 2018, 509, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ren, S.; Zhao, Q.; Lu, R.; Hang, C.; Chen, Z.; Zheng, H. Selective separation of methyl orange from water using magnetic ZIF-67 composites. Chem. Eng. J. 2018, 333, 49–57. [Google Scholar] [CrossRef]
- Zhou, L.; Jin, J.; Liu, Z.; Liang, X.; Shang, C. Adsorption of acid dyes from aqueous solutions by the ethylenediamine-modified magnetic chitosan nanoparticles. J. Hazard. Mater. 2011, 185, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- McMullan, G.; Meehan, C.; Conneely, A.; Kirby, N.; Robinson, T.; Nigam, P.; Banat, I.M.; Marchant, R.; Smyth, W.F. Microbial decolourisation and degradation of textile dyes. Appl. Microbiol. Biot. 2001, 56, 81–87. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.; Li, X.; Xiao, L. Visible light photocatalytic decolourization of C. I. acid red 66 by chitosan capped CdS composite nanoparticles. Chem. Eng. J. 2009, 152, 537–542. [Google Scholar] [CrossRef]
- Türgay, G.E.; Atalay, S.; Forss, J.; Welander, U. The treatment of azo dyes found in textile industry wastewater by anaerobic biological method and chemical oxidation. Sep. Purif. Technol. 2011, 79, 26–33. [Google Scholar] [CrossRef]
- Kositzi, M.; Poulios, I.; Samara, K.; Tsatsaroni, E.; Darakas, E. Photocatalytic oxidation of Cibacron Yellow LS-R. J. Hazard. Mater. 2007, 146, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Merzouk, B.; Yakoubi, M.; Zongo, I.; Leclerc, J.P.; Paternotte, G.; Pontvianne, S.; Lapicque, F. Effect of modification of textile wastewater composition on electrocoagulation efficiency. Desalination 2011, 275, 181–186. [Google Scholar] [CrossRef]
- Deepak, T.G.; Anjusree, G.S.; Pai, K.R.N.; Subash, D.; Nair, S.V.; Nair, A.S. Cabbage leaf-shaped two-dimensional TiO2 mesostructures for efficient dye-sensitized solar cells. RSC. Adv. 2014, 4, 27084–27090. [Google Scholar] [CrossRef]
- Nenavathu, B.P.; Krishna, A.V.R.; Goyal, A.R.; Kapoor, A.; Dutta, R.K. Synthesis, characterization and enhanced photocatalytic degradation efficiency of Se doped ZnO nanoparticles using trypan blue as a model dye. Appl. Catal. A: General. 2013, 459, 106–113. [Google Scholar] [CrossRef]
- Wahi, R.K.; Yu, W.W.; Liu, Y.; Mejia, M.L.; Falkner, J.C.; Nolte, W.; Colvin, V.L. Photodegradation of Congo Red catalyzed by nanosized TiO2. J. Mol. Catal. A: Chem. 2005, 242, 48–56. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Jin, B.; Chow, C.W.K.; Saint, C. Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem. Eng. J. 2009, 148, 354–364. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.H.; Jiang, J.J.; Wang, H.P.; Wei, Z.W.; Zhu, X.; Pan, M.; Su, C.Y. A stable metal cluster-metalloporphyrin MOF with high capacity for cationic dye removal. J. Mater. Chem. A 2018, 6, 17698–17705. [Google Scholar] [CrossRef]
- Li, J.; He, S.; Li, R.; Dai, W.; Tao, J.; Wang, C.; Liu, J.; Wu, T.; Tang, C. Template-free synthesis of three dimensional porous boron nitride nanosheets for efficient water cleaning. RSC. Adv. 2018, 8, 32886–32892. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Kamari, A.; Ngah, W.S.W.; Chong, M.Y.; Cheah, M.L. Sorption of acid dyes onto GLA and H2SO4 cross-linked chitosan beads. Desalination 2009, 249, 1180–1189. [Google Scholar] [CrossRef]
- Asuha, S.; Zhou, X.G.; Zhao, S. Adsorption of methyl orange and CrVI on mesoporous TiO2 prepared by hydrothermal method. J. Hazard. Mater. 2010, 181, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.; Maitra, S.; Ahmad, N.; Bustam, A.; Sen, T.K.; Dutta, B.K. Metal ion removal from aqueous solution using physic seed hull. J. Hazard. Mater. 2010, 179, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yu, N.; Wang, X.; Wang, Y.; Wang, L.; Li, X.; Hu, X. Adsorption Properties of Granular Activated Carbon-Supported Titanium Dioxide Particles for Dyes and Copper Ions. Sci. Rep. U.K. 2018, 8, 6463–6470. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xie, J.; Jiang, D.; Yan, Z.; Jing, J.; Liu, D. Enhanced adsorption of hydroxyl contained/anionic dyes on non functionalized Ni@SiO2 core–shell nanoparticles: Kinetic and thermodynamic profile. Appl. Surf. Sci. 2014, 292, 301–310. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination. 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, X.; Liu, S.; Zhou, L.; Li, W.; Zhang, J. Anionic Lanthanide Metal-Organic Frameworks: Selective Separation of Cationic Dyes, Solvatochromic Behavior, and Luminescent Sensing of Co(II) Ion. Inorg. Chem. 2018, 57, 11463–11473. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Fan, C.C.; Wei, Y.Z.; Du, J.; Zhu, H.B.; Zhao, Y. An anionic zeolite-like metal-organic framework (AZMOF) with a Moravia network for organic dye absorption through cation-exchange. Dalton Trans. 2016, 45, 10909–10915. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Rafatullah, M.; Sulaiman, O.; Ibrahim, M.H.; Hashim, R. Scavenging behaviour of meranti sawdust in the removal of methylene blue from aqueous solution. J. Hazard. Mater. 2009, 170, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: a review. Carbohyd. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Recent Advances in Graft Copolymerization and Applications of Chitosan: A Review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Sajab, M.S.; Chia, C.H.; Zakaria, S.; Jani, S.M.; Ayob, M.K.; Chee, K.L.; Khiew, P.S.; Chiu, W.S. Citric acid modified kenaf core fibers for removal of methylene blue from aqueous solution. Bioresource. Technol. 2011, 102, 7237–7243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gao, Q.; Luo, W.; Yan, C.; Ji, Z.; Duan, P. One-step synthesis of amino-functionalized attapulgite clay nanoparticles adsorbent by hydrothermal carbonization of chitosan for removal of methylene blue from wastewater. Coll. Surf. A: Physicochem. Eng. Asp. 2015, 470, 248–257. [Google Scholar] [CrossRef]
- Fan, H.; Zhou, L.; Jiang, X.; Huang, Q.; Lang, W. Adsorption of Cu2+ and methylene blue on dodecyl sulfobetaine surfactant-modified montmorillonite. Appl Clay Sci. 2014, 95, 150–158. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, C.; Xu, W. Magnetic Dendritic Materials for Highly Efficient Adsorption of Dyes and Drugs. ACS Appl. Mater. Inter. 2010, 2, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Arino, A. Magnetic polyoxometalates: from molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hamanaka, S.; Nishimoto, Y.; Irle, S.; Yokoyama, T.; Yoshikawa, H.; Awaga, K. In operando X-ray absorption fine structure studies of polyoxometalate molecular cluster batteries: polyoxometalates as electron sponges. J. Am. Chem. Soc. 2012, 134, 4918–4924. [Google Scholar] [CrossRef] [PubMed]
- Kourasi, M.; Wills, R.G.A.; Shah, A.A.; Walsh, F.C. Heteropolyacids for fuel cell applications. Electrochimica Acta. 2014, 127, 454–466. [Google Scholar] [CrossRef]
- Sha, J.; Zhu, P.; Yang, X.; Li, X.; Li, X.; Yue, M.; Zhou, K. Polyoxometalates Templated Metal Ag-Carbene Frameworks Anodic Material for Lithium-Ion Batteries. Inorg. Chem. 2017, 56, 11998–12002. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Yang, G.Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Nyman, M.; Burns, P.C. A comprehensive comparison of transition-metal and actinyl polyoxometalates. Chem. Soc. Rev. 2012, 41, 7354–7367. [Google Scholar] [CrossRef] [PubMed]
- Ammam, M. Polyoxometalates: formation, structures, principal properties, main deposition methods and application in sensing. J. Mater. Chem. A. 2013, 1, 6291–6312. [Google Scholar] [CrossRef]
- Chen, W.; Huang, L.; Hu, J.; Li, T.; Jia, F.; Song, Y.F. Connecting carbon nanotubes to polyoxometalate clusters for engineering high-performance anode materials. Phys. Chem. chem phys. 2014, 16, 19668–19673. [Google Scholar] [CrossRef] [PubMed]
- Kortz, U.; Müller, A.; van Slageren, J.; Schnack, J.; Dalal, N.S.; Dressel, M. Polyoxometalates: Fascinating structures, unique magnetic properties. Coord. Chem. Rev. 2009, 253, 2315–2327. [Google Scholar] [CrossRef]
- Yang, P.; Kortz, U. Discovery and Evolution of Polyoxopalladates. Account. Chem. Res. 2018, 51, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Uchida, S.; Uehara, K. Hierarchical design of nanostructured materials based on polyoxometalates. Pure. Appl. Chem. 2009, 81, 2369–2376. [Google Scholar] [CrossRef]
- Wang, Y.F.; Neyman, A.; Arkhangelsky, E.; Gitis, V.; Meshi, L.; Weinstock, I.A. Self-Assembly and Structure of Directly Imaged Inorganic-Anion Monolayers on a Gold Nanoparticle. J. Am. Chem. Soc. 2009, 131, 17412–17422. [Google Scholar]
- Tessonnier, J.P.; Goubert-Renaudin, S.; Alia, S.; Yan, Y.; Barteau, M.A. Structure, stability, and electronic interactions of polyoxometalates on functionalized graphene sheets. Langmuir. 2013, 29, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Oms, A.D.; Mialane, P. Diversity in structures and properties of 3d-incorporating polyoxotungstates. Chem. Soc. Rev. 2012, 41, 7497–7536. [Google Scholar] [CrossRef] [PubMed]
- Rosnes, M.H.; Musumeci, C.M.; Pradeep, C.P.; Mathieson, J.S.; Long, D.-L.; Song, Y.-F.; Pignataro, B.; Richard, C.; Cronin, L. Assembly of modular asymmetric organic-inorganic polyoxometalate hybrids into anisotropic nanostructures. J. Am. Chem. Soc. 2010, 132, 15490–15492. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.M.; Wales, D.J.; Newton, G.N. Shining a light on the photo-sensitisation of organic-inorganic hybrid polyoxometalates. Dalton Trans. 2018, 47, 5120–5136. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Wang, W.; Hao, J. Synthesis of organic-inorganic hybrid compounds and their self-assembled behavior in different solvents. J. Colloid. Interf. Sci. 2018, 519, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.Y.; Guo, D.; He, C.; Meng, Q.J. Crystal structures and properties of large protonated water clusters encapsulated by metal-organic frameworks. J. Am. Chem. Soc. 2009, 132, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.G.; Zhang, P.P.; Peng, J.; Meng, H.X.; Wang, X.; Zhu, M.; Wang, D.D.; Meng, C.I.; Alimaje, K. Organic–inorganic hybrids constructed from mixed-valence multinuclear copper complexes and templated by keggin polyoxometalates. Cryst. Growth Des. 2012, 12, 1273–1281. [Google Scholar] [CrossRef]
- Liu, H.S.; Lan, Y.Q.; Li, S.L. Metal−organic frameworks with diverse structures constructed by using capsule-like ligand and NiII based on ionothermal and hydrothermal methods. Cryst. Growth Des. 2010, 10, 5221–5226. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, B.; Sun, Y.; Tantai, X.; Xiao, X.; Wang, J.; Zhang, L. Construction of polyoxometallate-based organic-inorganic hybrid nanowires for efficient oxidative desulfurization. Mol. Catal. 2018, 448, 38–45. [Google Scholar] [CrossRef]
- Jia, H.; Li, Q.; Bayaguud, A.; She, S.; Huang, Y.; Chen, K.; Wei, Y. Tosylation of alcohols: An effective strategy for the functional group transformation of organic derivatives of polyoxometalates. Sci. Rep. U.K. 2017, 7, 12523–12531. [Google Scholar] [CrossRef] [PubMed]
- Nasim Khan, R.N.; Mahmood, N.; Lv, C.; Sima, G.; Zhang, J.; Hao, J.; Hou, Y.; Wei, Y. Pristine organo-imido polyoxometalates as an anode for lithium ion batteries. RSC. Adv. 2014, 4, 7374–7379. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, J.; Wang, P.; Ding, B.; Huang, Y.; Zhao, Z.; Zhang, J.; Wei, Y. Step-by-step strategy from achiral precursors to polyoxometalates-based chiral organic-inorganic hybrids. Inorg. Chem. 2015, 54, 2551–2559. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z. Rational synthesis of covalently bonded organic-inorganic hybrids. Angew. Chem. 2004, 43, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Li, Y.; Bi, Z.; Veith, G.M.; Bridges, C.A.; Guo, B.; Chen, J.; Mullins, D.R.; Surwade, S.P.; Mahurin, S.M.; et al. A POM–organic framework anode for Li-ion battery. J. Mater. Chem. A. 2015, 3, 22989–22995. [Google Scholar] [CrossRef]
- Genovese, M.; Lian, K. Polyoxometalate modified inorganic–organic nanocomposite materials for energy storage applications: A review. Curr. Opin. Solid. St. M. 2015, 19, 126–137. [Google Scholar] [CrossRef]
- Dolbecq, E.; Dumas, C.R. Mayer; P.; Mialane, Hybrid Organic-Inorganic Polyoxometalate Compounds: From Structural Diversity to Applications. Chem. Rev. 2010, 110, 6009–6048. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.R.; Chen, W.C.; Zhao, L.; Shao, K.Z.; Wang, X.L.; Su, Z.M. Functionalized polyoxometalate-based metal-organic cuboctahedra for selective adsorption toward cationic dyes in aqueous solution. Dalton Trans. 2018, 47, 12979–12983. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.X.; Yao, S.; Yang, Y.G.; Zhang, Z.M.; Lu, Y.; Chen, W.L.; Wang, E.B. Incorporating polyoxometalates into a porous MOF greatly improves its selective adsorption of cationic dyes. Chem. Eur. J. 2014, 20, 6927–6933. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Zhang, L.C.; Wang, Z.J.; Wang, L. Three new extended Preyssler-type polyoxometalates modified by transition metal-2,2′-biimidazole complexes. J. Solid State. Chem. 2012, 194, 207–276. [Google Scholar] [CrossRef]
- Zhang, P.P.; Peng, J.; Pang, H.J.; Sha, J.Q.; Zhu, M.; Wang, D.D.; Liu, M.G. The fastors affecting on the assembly of Ag-H2biim system: size, charge or shape of polyanions? CrystEngComm 2011, 13, 3832–3841. [Google Scholar] [CrossRef]
- Li, Z.L.; Wang, Y.; Zhang, L.C.; Wang, J.P.; You, W.S.; Zhu, Z.M. Three molybdophosphates based on Strandberg-type anions and Zn(II)-H2biim/H2O subunits: syntheses, structures and catalytic properties. Dalton Trans. 2014, 43, 5840–5846. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Xue, Q.; Dong, X.; Zhang, Y.; Hu, H.; Liu, B.; Xue, G. Three organic-inorganic hybrids based on [MoxOy]n- chains decorated with organic ligands and transition-metal coordination complexes. Eur. J. Inorg.Chem. 2017, 2017, 3516–3524. [Google Scholar] [CrossRef]
- Jacques, M. N,N-Dimethylformamide: much more than a solvent. Tetrahedron 2009, 65, 8313–8323. [Google Scholar]
- Mario, F.; Eduardo, R. Unexpected reactivity of graphene oxide with DUB and DMF. J. Mater. Chem. A 2018, 6, 12637–12646. [Google Scholar]
- Feng, M.; Zhao, G.; Gao, H.; Zhang, S. Tetracarboxyl-functionalized ionic liquid: Synthesis and catalytic properties. Aust. J. Chem. 2015, 68, 1513–1517. [Google Scholar] [CrossRef]
- Liu, C.G.; Zheng, T.; Liu, S.; Zhang, H.Y. Photodegradation of malachite green dye catalyzed by Keggin-type polyoxometalates under visible-light irradiation: Transition metal substituted effects. J. Mol. Struct. 2016, 1110, 44–52. [Google Scholar] [CrossRef]
- Liu, X.; Gong, W.; Luo, J.; Zou, C.; Yang, Y.; Yang, S. Selective adsorption of cationic dyes from aqueous solution by polyoxometalate-based metal–organic framework composite. Appl. Surf. Sci. 2016, 362, 517–524. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.M.; Zhao, S.Y.; Liu, P.; Wei, C.; Wu, Y.L.; Xia, C.K.; Xie, J.M. Three N–H functionalized metal-organic frameworks with selective CO2 uptake, dye capture, and catalysis. Inorg. Chem. 2014, 53, 7692–7699. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, B.; Lv, J.H.; Yu, K.; Wang, L.; Zhang, H.; Wang, S.; Zhou, B.B. One-step synthesis of two Wells–Dawson arsenotungstate hybrids via M–O–M bridges for efficient adsorption and selective separation of organic pollutants. CrystEngComm 2017, 19, 5653–5661. [Google Scholar] [CrossRef]
- Qu, S.; Huang, F.; Yu, S.; Chen, G.; Kong, J. Magnetic removal of dyes from aqueous solution using multi-walled carbon nanotubes filled with Fe2O3 particles. J. hazard. Mater. 2008, 160, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.D.; Qiu, L.G.; Jiang, X.Y.; Zhu, J.; Ye, S.; Jiang, X. Magnetic porous carbons with high adsorption capacity synthesized by a microwave-enhanced high temperature ionothermal method from a Fe-based metal-organic framework. Carbon. 2013, 59, 372–382. [Google Scholar] [CrossRef]
- Yi, F.Y.; Zhu, W.; Dang, S.; Li, J.P.; Wu, D.; Li, Y.H.; Sun, Z.M. Polyoxometalates-based heterometallic organic-inorganic hybrid materials for rapid adsorption and selective separation of methylene blue from aqueous solutions. Chem. Commun. 2015, 51, 3336–3339. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Tan, J.; Wang, C.; Wu, J.; Wang, Q.; Chen, J.; Fang, S.; Duan, M. A miniaturized evanescent-wave free chlorine sensor based on colorimetric determination by integrating on optical fiber surface. Sens. Actuat. B: Chem. 2017, 245, 674–682. [Google Scholar] [CrossRef]
- Guimarães Gusmão, K.A.; Alves Gurgel, L.V.; Sacramento Melo, T.M. Application of succinylated sugarcane bagasse as adsorbent to remove methylene blue and gentian violet from aqueous solutions -Kinetic and equilibrium studies. Dyes Pigments 2012, 92, 967–974. [Google Scholar] [CrossRef]
- Ding, B.B.; Weng, Y.Q.; Mao, Z.W.; Lam, C.K.; Chen, X.M.; Ye, B.H. Pillared-layer microporous metal-organic frameworks constructed by robust hydrogen bonds. synthesis, characterization, and magnetic and adsorption properties of 2,2-biimidazole and carboxylate complexes. Inorg. Chem. 2005, 44, 8836–8845. [Google Scholar] [CrossRef] [PubMed]






| Materials | Adsorption Capacity (mg/g) | Time (hrs) | Ref |
|---|---|---|---|
| Zn-DDQ a | 135 | 8 | [77] |
| C12H64As2Cu3N12O69W18 b | 320.5 | 1.5 | [78] |
| MWCNTs/Fe2O3 c | 42.3 | 1 | [79] |
| graphene oxide sponges | 397 | 1 | [80] |
| PW11V@MIL-101 d | 371 | 0.5 | [67] |
| ErCu–POM (Er-3) e | 391.3 | 0.5 | [81] |
| Compound 1 | 174.3 | 2 | This work |
| Compound 2 | 488.2 | 2 | This work |
| Compound 3 | 521.7 | 2 | This work |
| Compound 4 | 425.8 | 2 | This work |
| Compound 5 | 319.7 | 2 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Nie, Y.-M.; Li, S.-H.; Zhou, J.-L.; Yan, J. A Comprehensive Study on the Dye Adsorption Behavior of Polyoxometalate-Complex Nano-Hybrids Containing Classic β-Octamolybdate and Biimidazole Units. Molecules 2019, 24, 806. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24040806
Liang S, Nie Y-M, Li S-H, Zhou J-L, Yan J. A Comprehensive Study on the Dye Adsorption Behavior of Polyoxometalate-Complex Nano-Hybrids Containing Classic β-Octamolybdate and Biimidazole Units. Molecules. 2019; 24(4):806. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24040806
Chicago/Turabian StyleLiang, Shuang, Yan-Mei Nie, Sang-Hao Li, Jian-Liang Zhou, and Jun Yan. 2019. "A Comprehensive Study on the Dye Adsorption Behavior of Polyoxometalate-Complex Nano-Hybrids Containing Classic β-Octamolybdate and Biimidazole Units" Molecules 24, no. 4: 806. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24040806