Beer Molecules and Its Sensory and Biological Properties: A Review
Abstract
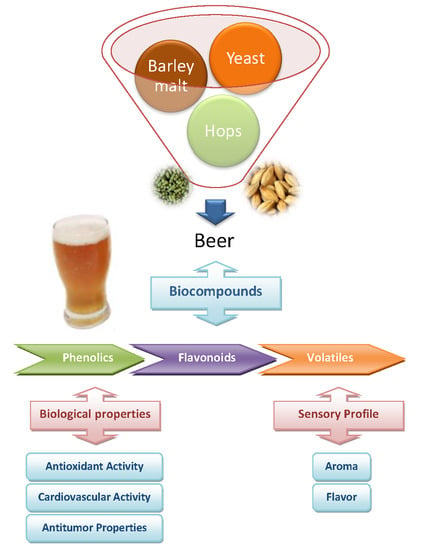
:1. Introduction
2. The Brewing Process
3. Beer Volatile Esters
4. Complementary Beer Volatile Compounds
4.1. Higher Alcohols
4.2. Carbonyl Compounds
5. Beer Phenolic Compounds Profile
6. Beer Compounds and Human Health
6.1. Antitumor Properties and Cancer Chemopreventive Potential Subsection
6.2. Anti-Inflammatory and Antioxidant Properties
6.3. Cardiovascular Diseases
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Defernez, M.; Foxall, R.J.; O’Malley, C.J.; Montague, G.; Ring, S.M.; Kemsley, E.K. Modelling beer fermentation variability. J. Food Eng. 2007, 83, 167–172. [Google Scholar] [CrossRef]
- Grassi, S.; Amigo, J.M.; Lyndgaard, C.B.; Foschino, R.; Casiraghi, E. Beer fermentation: Monitoring of process parameters by FT-NIR and multivariate data analysis. Food Chem. 2014, 155, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Hager, A.S.; Taylor, J.P.; Waters, D.M.; Arendt, E.K. Gluten free beer—A review. Trends Food Sci. Technol. 2014, 36, 44–54. [Google Scholar] [CrossRef]
- Osburn, K.; Amaral, J.; Metcalf, S.R.; Nickens, D.M.; Rogers, C.M.; Sausen, C.; Caputo, R.; Miller, J.; Li, H.; Tennessen, J.M.; et al. Primary souring: A novel bacteria-free method for sour beer production. Food Microbiol. 2018, 70, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Grassi, S.; Amigo, J.M.; Lyndgaard, C.B.; Foschino, R.; Casiraghi, E. Assessment of the sugars and ethanol development in beer fermentation with FT-IR and multivariate curve resolution models. Food Res. Int. 2014, 62, 602–608. [Google Scholar] [CrossRef]
- Mangindaan, D.; Khoiruddin, K.; Wenten, G. Beverage dealcoholization processes: Past, present, and future. Trends Food Sci. Technol. 2018, 71, 36–45. [Google Scholar] [CrossRef]
- Holt, S.; Mukherjee, V.; Lievens, B.; Verstrepen, K.J.; Thevelein, J.M. Bioflavoring by non-conventional yeasts in sequential beer fermentations. Food Microbiol. 2018, 72, 55–66. [Google Scholar] [CrossRef]
- Mastanjevic, K.; Sarkanj, B.; Krska, R.; Sulyok, M.; Warth, B.; Mastanjevic, K.; Santek, B.; Krstanovic, V. From malt to wheat beer: A comprehensive multi-toxin screening, transfer assessment and its influence on basic fermentation parameters. Food Chem. 2018, 254, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Shimizu, H.; Shioya, S. Beer Volatile Compounds and Their Application to Low-Malt Beer Fermentation. J. Biosci. Bioeng. 2008, 106, 317–323. [Google Scholar] [CrossRef]
- Dack, R.E.; Black, G.W.; Koutsidis, G.; Usher, S.J. The effect of Maillard reaction products and yeast strain on the synthesis of key higher alcohols and esters in beer fermentations. Food Chem. 2017, 232, 595–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodman, A.D.; Gerogiorgis, D.I. Dynamic optimization of beer fermentation: Sensitivity analysis of attainable performance vs. product flavor constraints. Comput. Chem. Eng. 2017, 106, 582–595. [Google Scholar] [CrossRef]
- Stefanuto, P.H.; Perrault, K.A.; Dubois, L.M.; L’Homme, B.; Allen, C.; Loughnane, C.; Ochiai, N.; Focant, J.F. Advanced method optimization for volatile aroma profiling of beer using two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2017, 1507, 45–52. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Chua, J.Y.; Liu, S.Q. Impact of simultaneous fermentation with Saccharomyces cerevisiae and Torulasporadelbrueckii on volatile and non-volatile constituents in beer. LWT Food Sci. Technol. 2018, 91, 26–33. [Google Scholar] [CrossRef]
- Ocvirk, M.; Mlinaric, N.K.; Kosir, I.J. Comparison of sensory and chemical evaluation of lager beer aroma by gas chromatography and gas chromatography/mass spectrometry. J. Sci. Food Agric. 2018, 80, 3627–3635. [Google Scholar] [CrossRef] [PubMed]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.C.; da Silva, A.A.S.; da Silva, L.S.N.; Godoy, R.L.O.; Nogueira, L.C.; Quitério, S.L.; Raices, S.L. Method development by GC–ECD and HS-SPME–GC–MS for beer volatile analysis. Food Chem. 2015, 167, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, P.; Kordialik-Bogacka, E. Alternatives to malt in brewing. Trends Food Sci. Technol. 2017, 65, 1–9. [Google Scholar] [CrossRef]
- Van Donkelaar, L.H.G.; Mostert, J.; Zisopoulos, F.K.; Boom, R.M.; Van Der Goot, A.J. The use of enzymes for beer brewing: Thermodynamic comparison on resource use. Energy 2016, 115, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Gous, P.W.; Fox, G.P. Review: Amylopectin synthesis and hydrolysis—Understanding isoamylase and limit dextrinase and their impact on starch structure on barley (Hordeum vulgare) quality. Trends Food Sci. Technol. 2017, 62, 23–32. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Innovative beer-brewing of typical, old and healthy wheat varieties to boost their spreading. J. Clean. Prod. 2018, 171, 297–311. [Google Scholar] [CrossRef]
- Mezgebe, A.G.; Abegaz, K.; Taylor, J.R.N. Relationship between waxy (high amylopectin) and high protein digestibility traits in sorghum and malting quality. J. Cereal Sci. 2018, 79, 319–327. [Google Scholar] [CrossRef]
- Farzaneh, V.; Ghodsvali, A.; Bakhshabadi, H.; Zare, Z.; Carvalho, I.S. The impact of germination time on the some selected parameters through malting process. Int. J. Biol. Macromol. A 2018, 94, 663–668. [Google Scholar] [CrossRef]
- Rodrigo, S.; Young, S.D.; Cook, D.; Wilkinson, S.; Clegg, S.; Bailey, E.H.; Mathers, A.W.; Broadley, M.R. Selenium in commercial beer and losses in the brewing process from wheat to beer. Food Chem. 2015, 182, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosinska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries—The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kerpes, R.; Fischer, S.; Becker, T. The production of gluten-free beer: Degradation of hordeins during malting and brewing and the application of modern process technology focusing on endogenous malt peptidases. Trends Food Sci. Technol. 2017, 67, 129–138. [Google Scholar] [CrossRef]
- Blanco, C.A.; Rojas, A.; Caballero, P.A.; Ronda, F.; Gomez, I.; Caballero, I. A better control of beer properties by predicting acidity of hop iso-α-acids. Trends Food Sci. Technol. 2006, 17, 373–377. [Google Scholar] [CrossRef]
- Caballero, I.; Blanco, C.A.; Porras, M. Iso-α-acids, bitterness and loss of beer quality during storage. Trends Food Sci. Technol. 2012, 26, 21–30. [Google Scholar] [CrossRef]
- Oladokun, O.; Tarrega, A.; James, S.; Smart, K.; Hort, J.; Cook, D. The impact of hop bitter acid and polyphenol profiles on the perceived bitterness of beer. Food Chem. 2016, 205, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Oladokun, O.; James, S.; Cowley, T.; Dehrmann, F.; Smart, K.; Hort, J.; Cook, D. Perceived bitterness character of beer in relation to hop variety and the impact of hop aroma. Food Chem. 2017, 230, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Vivian, A.F.; Aoyagui, C.T.; Oliveira, D.N.; Catharino, R.R. Mass spectrometry for the characterization of brewing process. Food Res. Int. 2016, 89, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Giovenzana, V.; Beghi, R.; Guidetti, R. Rapid evaluation of craft beer quality during fermentation process by vis/NIR spectroscopy. J. Food Eng. 2014, 142, 80–86. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Pérez-Carrillo, E.; Serna-Saldívar, S.O. Maltose and glucose utilization during fermentation of barley and sorghum lager beers as affected by β-amylase or amyloglucosidase addition. J. Cereal Sci. 2014, 60, 602–609. [Google Scholar] [CrossRef]
- Yu, W.; Quek, W.P.; Li, C.; Gilbert, R.G.; Fox, G.P. Effects of the starch molecular structures in barley malts and rice adjuncts on brewing performance. Fermentation 2018, 4, 103. [Google Scholar] [CrossRef]
- Pihlava, J.M.; Kuertelius, T. Determination of benzoxazinoids in wheat and rye beers by HPLC-DAD and UPLC-QTOF MS. Food Chem. 2016, 204, 400–408. [Google Scholar] [CrossRef]
- Mascia, I.; Fadda, C.; Karabin, M.; Dostálek, P.; Del Caro, A. Aging of craft durum wheat beer fermented with sourdough yeasts. LWT Food Sci. Technol. 2016, 65, 487–494. [Google Scholar] [CrossRef]
- Mayer, H.; Ceccaroni, D.; Marconi, O.; Sileoni, V.; Perretti, G.; Fantozzi, P. Development of an all rice malt beer: A gluten free alternative. LWT Food Sci. Technol. 2016, 67, 67–73. [Google Scholar] [CrossRef]
- Tokpohozin, S.E.; Thomas, W.J.F.; Fischer, S.; Becker, T. Polyphasic characterization of lactic acid bacteria isolated from Beninese sorghum beer starter. LWT Food Sci. Technol. 2017, 80, 51–58. [Google Scholar] [CrossRef]
- Hiralal, L.; Olaniran, A.O.; Pillay, B. Aroma-active ester profile of ale beer produced under different fermentation and nutritional conditions. J. Biosci. Bioeng. 2014, 117, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef]
- Renger, R.S.; Van Hateren, S.H.; Luyben, K.C.A.M. The formation of esters and higher alcohols during brewery fermentation: The effect of carbon dioxide pressure. J. Inst. Brew. 1992, 98, 509–513. [Google Scholar] [CrossRef]
- Van Rijswijck, I.M.H.; Wolkers-Rooijackers, J.C.M.; Abee, T.; Smid, E.J. Performance of non-conventional yeasts in co-culture with brewers’ yeast for steering ethanol and aroma production. Microb. Biotechnol. 2017, 10, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.; Liti, G. Saccharomyces pastorianus: Genomic insights inspiring innovation for industry. Yeast 2015, 32, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Nikulin, J.; Krogerus, K.; Gibson, B. Alternative Saccharomyces interspecies hybrid combinations and their potential for low-temperature wort fermentation. Yeast 2018, 35, 113–127. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Miró, P.; Serra-Cayuela, A.; Buxaderas, S.; López-Tamames, E. Assessment of the aroma profiles of low-alcohol beers using HS-SPME–GC-MS. Food Res. Int. 2014, 57, 196–202. [Google Scholar] [CrossRef]
- Kruis, A.J.; Levisson, M.; Mars, A.; Van Der Ploeg, M.; Daza, F.G.; Ellena, V.; Kengen, W.M.; Van Der Oost, J.; Weusthuis, R.A. Ethyl acetate production by the elusive alcohol acetyltransferase from yeast. Metab. Eng. 2017, 41, 92–101. [Google Scholar] [CrossRef]
- García, A.I.; García, L.A.; Díaz, M. Prediction of ester production in industrial beer fermentation. Enzyme Microb. Technol. 1994, 16, 66–71. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Mohtasebi, S.S.; Rodriguez-Mendez, M.L.; Lozano, J.; Ahmadi, R.H. Potential application of electronic nose technology in brewery. Trends Food Sci. Technol. 2011, 22, 165–174. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Pretorius, I.S.; Thevelein, J.M.; Delvaux, F.R. The Saccharomyces cerevisiae alcohol acetyl transferase geneATF1 is a target of the cAMP/PKA and FGM nutrient-signalling pathways. FEMS Yeast Res. 2003, 4, 285–296. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Van Laere, S.D.; Vanderhaegen, B.M.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Pretorius, I.S.; Thevelein, J.M.; Delvaux, F.R. Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl. Environ. Microbiol. 2003, 69, 5228–5237. [Google Scholar] [CrossRef]
- Ploier, B.; Korber, M.; Schmidt, C.; Koch, B.; Leitner, E.; Daum, G. Regulatory link between steryl ester formation and hydrolysis in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 977–986. [Google Scholar] [CrossRef]
- Klein, I.; Korber, M.; Athenstaedt, K.; Daum, G. The impact of nonpolar lipids on the regulation of the steryl ester hydrolases Tgl1p and Yeh1p in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 1491–1501. [Google Scholar] [CrossRef]
- Monerawela, C.; Bond, U. Brewing up a storm: The genomes of lager yeasts and how they evolved. Biotechnol. Adv. 2017, 45, 512–519. [Google Scholar] [CrossRef]
- Udeh, O. Role of magnesium ions on yeast performance during very high gravity fermentation. J. Brew. Distill. 2013, 4, 19–45. [Google Scholar] [CrossRef]
- De La Cueva, S.P.; Álvarez, J.; Végvári, Á.; Montilla-Gómez, J.; Cruz-López, O.; Delgado-Andrade, C.; Rufián-Henares, J.A. Relationship between HMF intake and SMF formation in vivo: An animal and human study. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Fukushige, T.; Yonezawa, T.; Sone, H. Genetic and physiological analysis of branched-chain alcohols and isoamyl acetate production in Saccharomyces cerevisiae. Appl. Microb. Biotechnol. 2001, 59, 501–508. [Google Scholar] [CrossRef]
- Takahashi, T.; Ohara, Y.; Sueno, K. Breeding of a sake yeast mutant with enhanced ethyl caproate productivity in sake brewing using rice milled at a high polishing ratio. J. Biosci. Bioeng. 2017, 123, 707–713. [Google Scholar] [CrossRef]
- Noba, S.; Yako, N.; Sakai, H.N.; Kobayashi, M.; Watanabe, T. Identification of a precursor of 2-mercapto-3-methyl-1-butanol in beer. Food Chem. 2017, 255, 282–289. [Google Scholar] [CrossRef]
- Kuzdralinski, A.; Solarska, E.; Muszynska, M. Deoxynivalenol and zearalenone occurence in beers analysed by an enzyme-linked immunosorbent assay method. Food Control 2013, 29, 22–24. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Rocha, L.O.; Fontes, L.C.; Carnielli, L.; Reis, T.A.; Corrêa, B. Mycotoxin analysis of industrial beers from Brazil: The influence of fumonisin B1 and deoxynivalenol in beer quality. Food Chem. 2017, 218, 64–69. [Google Scholar] [CrossRef]
- Pascari, X.; Ramos, A.J.; Marín, S.; Sanchís, V. Mycotoxins and beer. Impact of beer production process on mycotoxin contamination. A review. Food Res. Int. 2018, 103, 121–129. [Google Scholar] [CrossRef]
- Vidal, E.E.; Billerbeck, G.M.; Simões, D.A.; Schuler, A.; François, J.M.; Morais, M.A., Jr. Influence of nitrogen supply on the production of higher alcohols/esters and expression of flavor-related genes in cachaça fermentation. Food Chem. 2013, 138, 701–708. [Google Scholar] [CrossRef]
- Gallone, B.; Steensels, J.; Prahl, T.; Soriaga, L.; Saels, V.; Herrera-Malaver, B.; Merlevede, A.; Roncoroni, M.; Voordeckers, K.; Miraglia, L.; et al. Domestication and Divergence of Saccharomyces cerevisiae. Cell 2016, 166, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Bravi, E.; Benedetti, P.; Marconi, O.; Perrett, G. Determination of free fatty acids in beer wort. Food Chem. 2014, 151, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Magalhães, J.; Pereira, F.B.; Macieira, F.; Domingues, L.; Oliveira, J.M. Volatile fingerprinting diverse aged craft beers. LWT Food Sci. Technol. 2019, 108, 129–136. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Li, H.; Hao, J.; Jiang, W.; Liu, Z.; Qin, Q. Flavor contribution of esters in lager beer and an analysis of their flavor threshold. J. Am. Soc. Brew. Chem. 2017, 75, 201–206. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour threshold. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- Engan, B. Organoleptic threshold values of some alcohols and esters in beer. J. Inst. Brew. 1972, 78, 33–36. [Google Scholar] [CrossRef]
- Gutsche, K.A.; Tran, T.B.T.; Vogel, R.F. Production of volatile compounds by Lactobacillus sakei from branched chain α-keto acids. Food Microbiol. 2012, 29, 224–228. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Q.; Jin, H.; Zhang, X.; Xu, Y.; Yu, A.; Zhang, H.; Ding, L. Determination of xanthohumol in beer based on cloud point extraction coupled with high performance liquid chromatography. Talanta 2010, 81, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Schoondermark-Stolk, S.A.; Tabernero, M.; Chapman, J.; Ter Schure, E.G.; Verrips, C.T.; Verkleij, A.J.; Boonstra, J. Bat2p is essential in Saccharomyces cerevisiae for fusel alcohol production on the non-fermentable carbon source ethanol. FEMS Yeast Res. 2005, 5, 757–766. [Google Scholar] [CrossRef]
- Eden, A.; Simchen, G.; Benvenisty, N. Two yeast homologs of ECA39, a target for c-Myc regulation, code for cytosolic and mitochondrial branced-chain amino acid aminotransferases. J. Biol. Chem. 1996, 271, 20242–20245. [Google Scholar] [CrossRef]
- Minnaar, P.P.; Jolly, N.P.; Paulsen, V.; Plessis, W.D.; Van Der Rijst, M. Schizosaccharomyces pombe and Saccharomyces cerevisiae yeasts in sequential fermentations: Effect on phenolic acids of fermented Kei-apple (Dovyalis caffra L.) juice. Int. J. Food Microbiol. 2017, 257, 232–237. [Google Scholar] [CrossRef]
- Rosca, I.; Petrovici, A.R.; Brebu, M.; Stoica, I.; Minea, B.; Marangoci, N. An original method for producing acetaldehyde and diacetyl by yeast fermentation. Braz. J. Microbiol. 2016, 47, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Marri, L.; Jansson, A.M.; Christensen, C.E.; Hindsgaul, C.O. An enzyme-linked immunosorbent assay for the detection of diacetyl (2,3-butanedione). Anal. Biochem. 2017, 535, 12–18. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Niu, C.; Zheng, F.; Zhao, Y. Simultaneous determination of diethylacetal and acetaldehyde during beer fermentation and storage process. J. Sci. Food Agric. 2018, 98, 4733–4741. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kusaka, K.; Takahashi, T.; Sato, K. Method for the simultaneous assay of diacetyl and acetoin in the presence of alpha-acetolactate: Application in determining the kinetic parameters for the decomposition of alpha-acetolactate. J. Biosci. Bioeng. 2005, 99, 502–507. [Google Scholar] [CrossRef]
- Sone, H.; Fujii, T.; Kondo, K.; Shimizu, F.; Tanaka, J.; Inoue, T. Nucleotide sequence and expression of the Enterobacter aerogenes alpha-acetolactate decarboxylase gene in brewer’s yeast. Appl. Environ. Microbiol. 1988, 54, 38–42. [Google Scholar]
- Cejnar, R.; Hlozkova, K.; Kotrba, P.; Dostalek, P. Surface-engineered Saccharomyces cerevisiae displaying α-acetolactate decarboxylase from Acetobacter aceti ssp xylinum. Biotechnol. Lett. 2016, 38, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Dong, J.; Wu, D.; Chen, Y.; Guo, X.; Shi, Y.; Sun, X.; Xiao, D. Construction of recombinant industrial brewer’s yeast with lower diacetyl production and proteinase A activity. Eur. Food Res. Technol. 2012, 235, 951–961. [Google Scholar] [CrossRef]
- Shi, T.T.; Li, P.; Chen, S.J.; Chen, Y.F.; Guo, X.W.; Xiao, D.G. Reduced production of diacetyl by overexpressing BDH2 gene and ILV5 gene in yeast of the lager brewers with one ILV2 allelic gene deleted. J. Ind. Microbiol. Biotechnol. 2017, 44, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.T.; Guo, X.W.; Li, P.; Zhou, Z.; Xiao, D.G. Diacetyl content reduction in industrial brewer’s yeast through ILV2 disruption and BDH1 expression. Eur. Food Res. Technol. 2016, 242, 919–926. [Google Scholar] [CrossRef]
- Zapata, P.J.; Martinez-Esplá, A.; Gironés-Vilaplana, A.; Santos-Lax, D.; Noguera-Artiaga, L.; Carbonell-Barrachina, A.A. Phenolic, volatile, and sensory profiles of beer enriched by macerating quince fruits. LWT Food Sci. Technol. 2019, 103, 139–146. [Google Scholar] [CrossRef]
- Cheiran, K.P.; Raimundo, V.P.; Manfroi, V.; Anzanello, M.J.; Kahmann, A.; Rodrigues, E.; Frazzon, J. Simultaneous identification of low-molecular weight phenolic and nitrogen compounds in craft beers by HPLC-ESI-MS/MS. Food Chem. 2019, 286, 113–122. [Google Scholar] [CrossRef]
- Bettenhausen, H.M.; Barr, L.; Broeckling, C.D.; Chaparro, M.; Holbrook, C.; Sedin, D.; Heuberger, A.L. Influence of malt source on beer chemistry, flavor, and flavor stability. Food Res. Int. 2019, 113, 487–504. [Google Scholar] [CrossRef]
- De Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef]
- Moura-Nunes, N.; Brito, T.C.; da Fonseca, N.D.; de Aguiar, P.F.; Monteiro, M.; Perrone, D.; Torres, A.G. Phenolic compounds of Brazilian beers from different types and styles and application of chemometrics for modeling antioxidant capacity. Food Chem. 2016, 199, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Heuberger, A.L.; Broeckling, C.D.; Holbrook, C.; Barr, L.; Kirkpatrick, K.; Prenni, J.E. Evaluation of non-volatile metabolites in beer stored at high temperature and utility as an accelerated method to predict flavor stability. Food Chem. 2016, 200, 301–307. [Google Scholar] [CrossRef]
- Montanari, L.; Perretti, G.; Natella, F.; Guidi, A.; Fantozzi, P. Organic and phenolic acids in beer. LWT Food Sci. Technol. 1999, 32, 535–539. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010, 119, 1150–1158. [Google Scholar] [CrossRef]
- Waters, M.T.; Heasman, A.P.; Hughes, P.S. Comparison of (+)-catechin and ferulic acid as natural antioxidants and their impact on beer flavor stability. Part 1: Forced-aging. J. Am. Soc. Brew. Chem. 1997, 55, 83–89. [Google Scholar]
- Oliveira Neto, J.R.; Oliveira, T.S.; Ghedini, P.C.; Vaz, B.G.; Gil, E.S. Antioxidant and vasodilatory activity of commercial beers. J. Funct. Foods 2017, 34, 130–138. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Prorok, P.; Piórecki, N. Physicochemical and antioxidative properties of Cornelian cherry beer. Food Chem. 2019, 281, 147–153. [Google Scholar] [CrossRef]
- Andersson, A.A.M.; Lampi, A.-M.; Nystrom, L.; Piironen, V.; Li, L.; Ward, J.L.; Åman, P. Phytochemical and Dietary Fiber Components in Barley Varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9767–9776. [Google Scholar] [CrossRef]
- Khakimov, B.; Jespersen, B.; Engelsen, S. Comprehensive and Comparative Metabolomic Profiling of Wheat, Barley, Oat and Rye Using Gas Chromatography-Mass Spectrometry and Advanced Chemometrics. Foods 2014, 3, 569–585. [Google Scholar] [CrossRef]
- Kim, M.J.; Hyun, J.N.; Kim, J.A.; Park, J.C.; Kim, M.Y.; Kim, J.G.; Chung, I.M. Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J. Agric. Food Chem. 2007, 55, 4802–4809. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yanagida, A.; Komeya, S.; Kawana, M.; Honma, D.; Tagashira, M.; Shibusawa, Y. Comprehensive separation and structural analyses of polyphenols and related compounds from bracts of hops (Humulus lupulus L.). J. Agric. Food Chem. 2014, 62, 2198–2206. [Google Scholar] [CrossRef]
- Kim, H.J.; Yim, S.H.; Han, F.; Kang, B.Y.; Choi, H.J.; Jung, D.W.; Williams, D.R.; Gustafson, K.R.; Kennelly, E.J.; Lee, I.S. Biotransformed metabolites of the hop Prenylflavonone isoxanthohumol. Molecules 2019, 24, 394. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Oak, M.H.; Auger, C.; Belcastro, E.; Park, S.H.; Lee, H.H.; Schini-Kerth, V.B. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Magraner, E.; Cordines, X.; Valderas-Martínez, P.; Roth, I.; Arranz, S.; Casas, R.; Navarro, M.; Hervas, A.; Sisó, A.; et al. Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: A randomized feeding Trial. Nutr. Metabol. Cardiovasc. Dis. 2015, 25, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Seliger, J.M.; Cicek, S.S.; Witt, L.T.; Martin, H.J.; Masser, E.; Hintzpeter, J. Selective inhibition of human AKR1B10 by n-humulone, Adhumulone and Cohumulone isolated from Humulus lupulus extract. Molecules 2018, 23, 3041. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albanese, L.; Di Stefano, V.; Delisi, R.; Avellone, G.; Meneguzzo, F.; Pagliaro, M. Beer produced via hydrodynamic cavitation retains higher amounts of xanthohumol and other hops prenylflavonoids. LWT Food Sci. Technol. 2018, 91, 160–167. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, R. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with Saccharomyces cerevisiae for the production of craft beers with potential health value added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef]
- León-Gonzales, M.E.; Gómez-Mejía, E.; Rosales-Conrado, N.; Madrid-Albarrán, Y. Residual brewing yeast as a source of polyphenols: Extraction, identification and quantification by chromatographic and chemometric tools. Food Chem. 2018, 267, 246–254. [Google Scholar] [CrossRef]



| Beer Compound | Concentration Found in Beer 1 (ppm) | Perception Threshold 2 (ppm) | References |
|---|---|---|---|
| Esters | |||
| Ethyl acetate | 15.3–16.8 | 5–10; 25–50 | [16,39,40,64,65,66] |
| Phenyl ethyl acetate | 0.1–0.73 | 3–5 | [39,44,64,65,67] |
| Isoamyl acetate | 0.078–0.489; 1.2 | 0.03; 1–2.5 | [16,39,44,64,66] |
| Isobutyl acetate | 0.03–1.2 | 0.5–1 | [65,67] |
| Ethyl caproate (ethyl hexanoate) | 0.081–0.411 | 0.014–0.2; 0.2–0.3 | [16,39,44,64,66,67] |
| Ehtyloctanoate | 0.04–0.53 | 0.9 | [44,65,67] |
| Higher alcohol | |||
| Amyl alcohol | 8.73–44 | 50–70 | [40,66,67] |
| Isobutyl alcohol | 6.6; 58.9 | 100–175 | [44,66,67] |
| Carbonyl compounds | |||
| Acetaldehyde | 0.952–8.1 | 1.114–5 | [66,67,73] |
| Diacetyl | 0.013–0.07 | 0.1–0.2 | [16,66,67,73,74] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonça, M.d.C.; Padilha, F.F. Beer Molecules and Its Sensory and Biological Properties: A Review. Molecules 2019, 24, 1568. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24081568
Humia BV, Santos KS, Barbosa AM, Sawata M, Mendonça MdC, Padilha FF. Beer Molecules and Its Sensory and Biological Properties: A Review. Molecules. 2019; 24(8):1568. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24081568
Chicago/Turabian StyleHumia, Bruno Vieira, Klebson Silva Santos, Andriele Mendonça Barbosa, Monize Sawata, Marcelo da Costa Mendonça, and Francine Ferreira Padilha. 2019. "Beer Molecules and Its Sensory and Biological Properties: A Review" Molecules 24, no. 8: 1568. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24081568