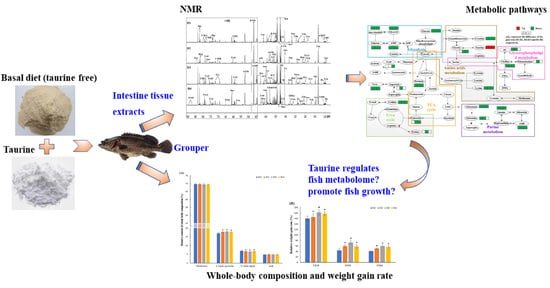
Metabolic Effect of Dietary Taurine Supplementation on Grouper (Epinephelus coioides): A 1H-NMR-Based Metabolomics Study
Abstract
:1. Introduction
2. Results
2.1. Growth of Grouper under Taurine Supplementation
2.2. Metabolic Profiles of the Grouper Intestine
2.3. Metabolic Trajectory of Grouper Intestine During the Feed Period
2.4. Physiological and Metabolic Variations in Response to Different Taurine Contents
3. Discussion
3.1. Energy Metabolism
3.2. Amino Acid Metabolism
3.3. Purine Metabolism
3.4. Lipid and Glycerophospholipid Metabolism
4. Materials and Methods
4.1. Diet Preparation
4.2. Fish Rearing and Experimental Conditions
4.3. Sampling Procedure
4.4. Sample Preparation and 1H-NMR Spectroscopy
4.5. Preprocessing of NMR Spectra and Multivariate Analysis
4.6. Metabolic Pathways Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gedi, M.A.; Magee, K.J.; Darwish, R.; Eakpetch, P.; Young, I.; Gray, D.A. Impact of the partial replacement of fish meal with a chloroplast rich fraction on the growth and selected nutrient profile of zebrafish (Danio rerio). Food Funct. 2019, 10, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, N.; Klinger, D.H.; Sims, N.A.; Yoshioka, J.R.; Kittinger, J.N. Nutritional attributes, substitutability, scalability, and environmental intensity of an illustrative subset of current and future protein sources for aquaculture feeds: Joint consideration of potential synergies and trade-offs. Environ. Sci. Technol. 2018, 52, 5532–5544. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Colen, R.; Aragao, C. Supplementing taurine to plant-based diets improves lipid digestive capacity and amino acid retention of Senegalese sole (Solea senegalensis) juveniles. Aquaculture 2017, 468, 94–101. [Google Scholar] [CrossRef]
- Koven, W.; Peduel, A.; Gada, M.; Nixon, O.; Ucko, M. Taurine improves the performance of white grouper juveniles (Epinephelus Aeneus) fed a reduced fish meal diet. Aquaculture 2016, 460, 8–14. [Google Scholar] [CrossRef]
- Wang, J.; Tao, Q.Y.; Wang, Z.; Mai, K.S.; Xu, W.; Zhang, Y.J.; Ai, Q.H. Effects of fish meal replacement by soybean meal with supplementation of functional compound additives on intestinal morphology and microbiome of Japanese seabass (Lateolabrax japonicus). Aquac. Res. 2017, 48, 2186–2197. [Google Scholar] [CrossRef]
- Michelato, M.; Furuya, W.M.; Gatlin, D.M. Metabolic responses of Nile tilapia Oreochromis niloticus to methionine and taurine supplementation. Aquaculture 2018, 485, 66–72. [Google Scholar] [CrossRef]
- Millamena, O.M. Replacement of fish meal by animal by-product meals in a practical diet for grow-out culture of grouper Epinephelus coioides. Aquaculture 2002, 204, 75–84. [Google Scholar] [CrossRef]
- Al-Feky, S.S.A.; El-Sayed, A.F.M.; Ezzat, A.A. Dietary taurine enhances growth and feed utilization in larval Nile tilapia (Oreochromis niloticus) fed soybean meal-based diets. Aquac. Nutr. 2016, 22, 457–464. [Google Scholar] [CrossRef]
- Salze, G.P.; Davis, D.A. Taurine: A critical nutrient for future fish feeds. Aquaculture 2015, 437, 215–229. [Google Scholar] [CrossRef]
- Shen, G.P.; Huang, Y.; Dong, J.Y.; Wang, X.X.; Cheng, K.K.; Feng, J.H.; Xu, J.J.; Ye, J.D. Metabolic effect of dietary taurine supplementation on Nile Tilapia (Oreochromis nilotictus) evaluated by NMR-Based metabolomics. J. Agric. Food Chem. 2018, 66, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Poppi, D.A.; Moore, S.S.; Glencross, B.D. The effect of taurine supplementation to a plant-based diet for barramundi (Lates calcarifer) with varying methionine content. Aquac. Nutr. 2018, 24, 1340–1350. [Google Scholar] [CrossRef]
- Alipiah, N.M.; Ramli, N.H.S.; Low, C.F.; Shamsudin, M.N.; Yusoff, F.M. Protective effects of sea cucumber surface-associated bacteria against Vibrio harveyi in brown-marbled grouper fingerlings. Aquac. Environ. Interact. 2016, 8, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.H.; Cheng, M.Y. Effects of dietary organic acid supplementation on the growth, nutrient digestibility and intestinal histology of the giant grouper Epinephelus lanceolatus fed a diet with soybean meal. Aquaculture 2017, 469, 106–111. [Google Scholar] [CrossRef]
- Santos, J.F.; Soares, K.L.S.; Assis, C.R.D.; Guerra, C.A.M.; Lemos, D.; Carvalho, L.B.; Bezerra, R.S. Digestive enzyme activity in the intestine of Nile tilapia (Oreochromis niloticus L.) under pond and cage farming systems. Fish Physiol. Biochem. 2016, 42, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.A.; Batista, S.; Pires, M.A.; Silva, A.P.; Pereira, L.F.; Saavedra, M.J.; Ozorio, R.O.A.; Rema, P. Dietary probiotic supplementation improves growth and the intestinal morphology of Nile tilapia. Animal 2017, 11, 1259–1269. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Connelly, J.; Lindon, J.C.; Holmes, E. Metabonomics: A platform for studying drug toxicity and gene function. Nat. Rev. Drug Discov. 2002, 1, 153–161. [Google Scholar] [CrossRef]
- Cheng, K.; Mullner, E.; Moazzami, A.A.; Carlberg, H.; Brannas, E.; Pickova, J. Metabolomics approach to evaluate a baltic sea sourced diet for cultured Arctic Char (Salvelinus alpinus L.). J. Agric. Food Chem. 2017, 65, 5083–5090. [Google Scholar] [CrossRef]
- Tsiafoulis, C.G.; Papaemmanouil, C.; Alivertis, D.; Tzamaloukas, O.; Miltiadou, D.; Balayssac, S.; Malet-Martino, M.; Gerothanassis, I.P. NMR-based metabolomics of the lipid fraction of organic and conventional bovine milk. Molecules 2019, 24, 1067. [Google Scholar] [CrossRef]
- Chauton, M.S.; Galloway, T.F.; Kjorsvik, E.; Storseth, T.R.; Puvanendran, V.; van der Meeren, T.; Karlsen, O.; Ronnestad, I.; Hamre, K. 1H NMR metabolic profiling of cod (Gadus morhua) larvae: Potential effects of temperature and diet composition during early developmental stages. Biol. Open 2015, 4, 1671–1678. [Google Scholar] [CrossRef]
- Schock, T.B.; Newton, S.; Brenkert, K.; Leffler, J.; Bearden, D.W. An NMR-based metabolomic assessment of cultured cobia health in response to dietary manipulation. Food Chem. 2012, 133, 90–101. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.F.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0-the human metabolome database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Sunram-Lea, S.I. Metabolic agents that enhance ATP can improve cognitive functioning: A review of the evidence for glucose, oxygen, pyruvate, creatine, and L-carnitine. Nutrients 2011, 3, 735–755. [Google Scholar] [CrossRef] [PubMed]
- Salze, G.P.; Spangler, E.; Cobine, P.A.; Rhodes, M.; Davis, D.A. Investigation of biomarkers of early taurine deficiency in Florida pompano Trachinotus carolinus. Aquaculture 2016, 451, 254–265. [Google Scholar] [CrossRef]
- Shen, G.P.; Li, Z.S.; Zhang, Y.; Wu, H.F.; Feng, J.H. 1H NMR-based metabolomics study on the physiological variations during the rat pregnancy process. Mol. Cell. Endocrinol. 2016, 423, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sasaki, T.; Awa, M.; Inomata, M.; Honryo, T.; Agawa, Y.; Ando, M.; Sawada, Y. Effect of dietary taurine enhancement on growth and development in red sea bream Pagrus major larvae. Aquac. Res. 2016, 47, 1168–1179. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.S.; Trushenski, J.; Wu, G.Y. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef]
- Hasko, G.; Sitkovsky, M.V.; Szabo, C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol. Sci. 2004, 25, 152–157. [Google Scholar] [CrossRef]
- Schock, T.B.; Duke, J.; Goodson, A.; Weldon, D.; Brunson, J.; Leffler, J.W.; Bearden, D.W. Evaluation of pacific white shrimp (Litopenaeus vannamei) health during a superintensive aquaculture growout using NMR-based metabolomics. PLoS ONE 2013, 8, e59521. [Google Scholar] [CrossRef]
- Ye, J.D.; Chen, J.C.; Wang, K. Growth performance and body composition in response to dietary protein and lipid levels in Nile tilapia (Oreochromis niloticus Linnaeus, 1758) subjected to normal and temporally restricted feeding regimes. J. Appl. Ichthyol. 2016, 32, 332–338. [Google Scholar] [CrossRef]
- Zhang, C.; Lian, C.J.; Yang, Z.J.; Sun, S.C.; Zhou, S.S.; Niu, S.L. Effects of different fatty source on fatty liver performance and composition of fatty liver in landes goose. J. Anim. Vet. Adv. 2012, 11, 2964–2968. [Google Scholar]
- Cheng, K.; Wagner, L.; Moazzami, A.A.; Gomez-Requeni, P.; Vestergren, A.S.; Brannas, E.; Pickova, J.; Trattner, S. Decontaminated fishmeal and fish oil from the Baltic Sea are promising feed sources for Arctic char (Salvelinus alpinus L.) studies of flesh lipid quality and metabolic profile. J. Lipid Sci. Technol. 2016, 118, 862–873. [Google Scholar] [CrossRef]
- Hua, K.; Suwendi, E.; Bureau, D.P. Effect of body weight on lysine utilization efficiency in Nile Tilapia (Oreochromis niloticus). Aquaculture 2019, 505, 47–53. [Google Scholar] [CrossRef]
- Wu, H.F.; Southam, A.D.; Hines, A.; Viant, M.R. High-throughput tissue extraction protocol for NMR- and MS-based metabolomics. Anal. Biochem. 2008, 372, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Charlton, A.J.; Godward, J.; Jones, S.A.; Harrison, M.; Wilson, J.C. Adaptive binning: An improved binning method for metabolomics data using the undecimated wavelet transform. Chem. Intell. Lab. Syst. 2007, 85, 144–154. [Google Scholar] [CrossRef]
- Dieterle, F.; Schlotterbeck, G.T.; Ross, A.; Niederhauser, U.; Senn, H. Application of metabonomics in a compound ranking study in early drug development revealing drug-induced excretion of choline into urine. Chem. Res. Toxicol. 2006, 19, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Climaco-Pinto, R.; Barros, A.S.; Locquet, N.; Schmidtke, L.; Rutledge, D.N. Improving the detection of significant factors using ANOVA-PCA by selective reduction of residual variability. Anal. Chim. Acta 2009, 653, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 2014, 42, D199–D205. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.Z.; Bourque, G.; Wishart, D.S.; Xia, J.G. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
Sample Availability: Not available. |





| Metabolites | D1-D2 | D1-D3 | D1-D4 | D2-D3 | D2-D4 | D3-D4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2X = 0.762 a R2Y = 0.974 Q2 = 0.920 p = 0.000 | 0.738 0.979 0.908 0.000 | 0.807 0.972 0.910 0.000 | 0.668 0.852 0.209 1.000 | 0.703 0.949 0.779 0.007 | 0.763 0.876 0.259 0.1333 | |||||||
| Fold b | r c | Fold | r | Fold | r | Fold | r | Fold | r | Fold | r | |
| Energy metabolism | ||||||||||||
| α-Glucose | 0.628 | −0.780 | 0.603 | −0.810 | 0.573 | −0.760 | 0.865 | −0.724 | ||||
| β-Glucose | 0.589 | −0.778 | 0.553 | −0.824 | 0.567 | −0.792 | 0.803 | −0.742 | ||||
| Lactate | 1.205 | 0.500 | 0.796 | −0.560 | ||||||||
| NAD | 0.756 | −0.530 | 0.794 | −0.615 | 0.834 | −0.513 | ||||||
| Lipid metabolism | ||||||||||||
| Phosphocholine | 0.484 | −0.524 | 0.445 | −0.546 | ||||||||
| Choline | 0.646 | −0.560 | 0.492 | −0.660 | 0.591 | −0.672 | ||||||
| myo-Inositol | 0.482 | −0.769 | 0.416 | −0.801 | 0.418 | −0.797 | 1.175 | 0.502 | 0.782 | −0.508 | 0.761 | −0.520 |
| Glycerol | 0.636 | −0.677 | 0.625 | −0.669 | 0.587 | −0.742 | ||||||
| Ethanolamine | 0.637 | −0.717 | ||||||||||
| Amino acid metabolism | ||||||||||||
| Taurine | 1.316 | 0.947 | 1.298 | 0.862 | 1.359 | 0.943 | 1.023 | 0.499 | ||||
| Aspartate | 0.595 | −0.620 | 0.377 | −0.518 | ||||||||
| Asparagine | 1.339 | 0.518 | ||||||||||
| Glutamate | 0.667 | −0.770 | 0.694 | −0.643 | 0.575 | −0.823 | 0.823 | −0.591 | ||||
| Glutamine | 0.781 | −0.551 | 0.775 | −0.524 | ||||||||
| Lysine | 0.649 | −0.858 | 0.639 | −0.867 | 0.640 | −0.813 | ||||||
| Proline | 0.696 | −0.537 | ||||||||||
| Glycine | 0.573 | −0.645 | 0.517 | −0.713 | 0.451 | −0.539 | ||||||
| NAA | 0.642 | −0.760 | 0.534 | −0.823 | 0.613 | −0.552 | ||||||
| 1-Methylhistidine | 1.529 | 0.502 | ||||||||||
| Creatine | 0.816 | −0.741 | 0.804 | −0.759 | 0.763 | −0.825 | 0.916 | −0.533 | 0.955 | −0.623 | ||
| Inosine | 0.792 | −0.697 | 0.646 | −0.595 | 0.723 | −0.708 | 0.780 | −0.540 | ||||
| Other | ||||||||||||
| AMP | 0.563 | −0.719 | 0.497 | −0.737 | 0.507 | −0.750 | 1.707 | 0.561 | ||||
| Formate | 0.664 | −0.745 | 0.541 | −0.769 | 0.599 | −0.798 | ||||||
| Uridine | 0.691 | −0.698 | 0.587 | −0.712 | 0.638 | −0.721 | ||||||
| Fumarate | 0.723 | −0.645 | 0.726 | −0.700 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, G.; Wang, S.; Dong, J.; Feng, J.; Xu, J.; Xia, F.; Wang, X.; Ye, J. Metabolic Effect of Dietary Taurine Supplementation on Grouper (Epinephelus coioides): A 1H-NMR-Based Metabolomics Study. Molecules 2019, 24, 2253. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24122253
Shen G, Wang S, Dong J, Feng J, Xu J, Xia F, Wang X, Ye J. Metabolic Effect of Dietary Taurine Supplementation on Grouper (Epinephelus coioides): A 1H-NMR-Based Metabolomics Study. Molecules. 2019; 24(12):2253. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24122253
Chicago/Turabian StyleShen, Guiping, Shenghao Wang, Jiyang Dong, Jianghua Feng, Jingjing Xu, Feng Xia, Xuexi Wang, and Jidan Ye. 2019. "Metabolic Effect of Dietary Taurine Supplementation on Grouper (Epinephelus coioides): A 1H-NMR-Based Metabolomics Study" Molecules 24, no. 12: 2253. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24122253