Structure and Dynamics of the Superprotonic Conductor Caesium Hydrogen Sulfate, CsHSO4
Abstract
:1. Introduction
2. Results and Discussion
2.1. CsHSO4 Spectroscopy
2.2. CsDSO4 Spectroscopy
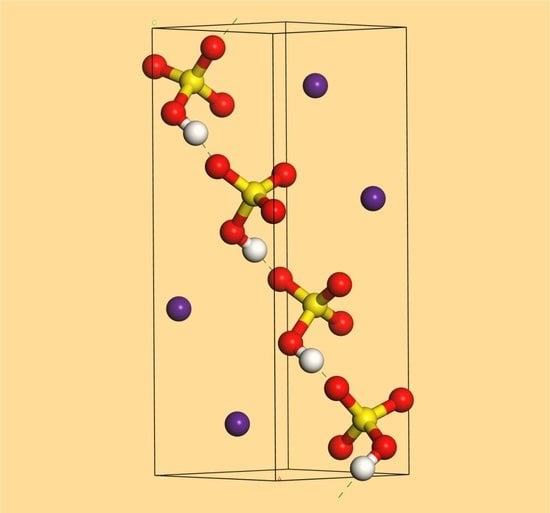
2.3. CsHSO4 Structure
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Norby, T. The promise of protonics. Nature 2001, 410, 877–878. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.M.; Boysen, D.A.; Chisholm, C.R.I.; Merle, R.B. Solid acids as fuel cell electrolytes. Nature 2001, 410, 910–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranowski, B.; Lipkowski, J.; Lunden, A. On the phase transitions of cesium hydrogen sulfate (CsHSO4). J. Solid State Chem. 1995, 117, 412–413. [Google Scholar] [CrossRef]
- Nozik, Y.Z.; Lyakhovitskaya, L.I.; Shchagina, N.M.; Sarin, V.A. Neutron diffraction study of crystal structures of I, II, and III phases of cesium hydrogen sulfate using the full-profile analysis technique. Kristallografiya 1990, 35, 658–660. [Google Scholar]
- Balagurov, A.M.; Belushkin, A.V.; Beskrovnyi, A.I.; Vratislav, S.; Wasicki, J.; Dutt, I.D.; Dlouha, M.; Jirak, Z.; Natkaniec, I.; Savenko, B.N.; et al. Crystal data and symmetry of phases of cesium hydrogen sulfate and selenate. JINR Rapid Commun. (Dubna Russia) 1985, 13–85, 18–28. [Google Scholar]
- Itoh, K.; Ukeda, T.; Ozaki, T.; Nakamura, E. Redetermination of the structure of caesium hydrogensulfate. Acta Crystallogr. C 1990, 46, 358–361. [Google Scholar] [CrossRef]
- Belushkin, A.V.; Adams, M.A.; Hull, S.; Shuvalov, L.A. P-T phase diagram of CsHSO4. Neutron scattering study of structure and dynamics. Solid State Ion. 1995, 77, 91–96. [Google Scholar] [CrossRef]
- Belushkin, A.V.; David, W.I.F.; Ibberson, R.M.; Shuvalov, L.A. High-resolution neutron powder diffraction studies of the structure of CsDSO4. Acta Crystallogr. B 1991, 47, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Jirák, Z.; Dlouhá, M.; Vratislav, S.; Balagurov, A.M.; Beskrovnyi, A.I.; Gordelii, V.I.; Datt, I.D.; Shuvalov, L.A. A neutron diffraction study of the superioic phase of CsHSO4. Phys. Status Solidi A 1987, 100, K117–K122. [Google Scholar] [CrossRef]
- Merinov, B.V.; Baranov, A.I.; Shuvalov, L.A.; Maksimov, B.A. Crystal structure of superionic phase CsDSO4 and phase transitions in cesium hydro-and deuterosulfates (selenates). Kristallografiya 1987, 32, 86–92. [Google Scholar]
- Merinov, B.V. Localization of hydrogen atoms in protonic conductors with a dynamically disordered network of hydrogen bonds: Effect of anomalous manifestation of hydrogen atoms on electron-density maps. Crystallogr. Rep. 1997, 42, 906–917. [Google Scholar]
- Yamawaki, H.; Fujihisa, H.; Sakashita, M.; Honda, K. Vibrational spectra of CsHSO4 at high pressure and high temperature. Phys. Rev. B 2007, 75, 094111. [Google Scholar] [CrossRef]
- Egami, T.; Billinge, S.J.L. Underneath the Bragg Peaks, Structural Analysis of Complex Materials, 2nd ed.; Pergamon: Oxford, UK, 2012. [Google Scholar]
- Bennett, E.L.; Murphy, P.J.; Imberti, S.; Parker, S.F. Characterisation of the hydrides in Stryker’s reagent: [HCu{P(C6H5)3}]6. Inorg. Chem. 2014, 53, 2963–2967. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.F.; Bowron, D.T.; Imberti, S.; Soper, A.K.; Refson, K.; Lox, E.S.; Lopez, M.; Albers, P. Structure determination of adsorbed hydrogen on real catalysts. Chem. Commun. 2010, 46, 2959–2961. [Google Scholar] [CrossRef] [PubMed]
- Zetterström, P.; Belushkin, A.V.; McGreevy, R.L.; Shuvalov, L.A. Structure and proton conduction in CsDSO4. Solid State Ion. 1999, 116, 321–329. [Google Scholar] [CrossRef]
- Belushkin, A.V.; Carlile, C.J.; Shuvalov, L.A. The diffusion of protons in the superionic conductor CsHSO4 by quasielastic neutron scattering. J. Phys. Condens. Matter 1992, 4, 389–398. [Google Scholar] [CrossRef]
- Ortiz, E.; Vargas, R.A.; Mellander, B.-E. Phase behaviour of the solid proton conductor CsHSO4. J. Phys. Condens. Matter 2006, 18, 9561–9573. [Google Scholar] [CrossRef]
- Dimitriev, V.P.; Loshkarev, V.V.; Rabin, L.M.; Shuvalov, L.A.; Yuzyuk, T.I. Combinational light-scattering and transition mechanisms in cesium hydrosulfate. Kristallografiya 1986, 31, 673–677. [Google Scholar]
- Baran, J.J. Polarized infrared and Raman spectra of a CsHSO4 single crystal. J. Mol. Struct. 1987, 162, 211–228. [Google Scholar] [CrossRef]
- Baran, J.; Marchewka, M.K. Vibrational investigation of phase transitions in CsHSO4 crystal. J. Mol. Struct. 2002, 614, 133–149. [Google Scholar] [CrossRef]
- Pham-Thi, M.; Colomban, P.; Novak, A.; Blinc, R. Phase transitions in superionic protonic conductors CsHSO4 and CsHSeO4. Solid State Commun. 1985, 55, 265–270. [Google Scholar] [CrossRef]
- Colomban, P.; Pham-Thi, M.; Novak, A. Thermal history and phase transitions in the superionic protonic conductors CsHSO4 and CsHSeO4. Solid State Ion. 1986, 20, 125–134. [Google Scholar] [CrossRef]
- Colomban, P.; Pham-Thi, M.; Novak, A. Influence of thermal and mechanical treatment and of water on structural phase transitions in CsHSO4. Solid State Ion. 1987, 24, 193–203. [Google Scholar] [CrossRef]
- Varma, V.; Rangavittal, N.; Rao, C.N.R. A study of superionic CsHSO4 and Cs1-xLixHSO4 by vibrational spectroscopy and X-ray diffraction. J. Solid State Chem. 1993, 106, 164–173. [Google Scholar] [CrossRef]
- Balagurov, A.M.; Belushkin, A.V.; Dutt, I.D.; Natkaniec, I.; Plakida, N.M.; Savenko, B.N.; Shuvalov, L.A.; Wasicki, J. Neutron scattering studies on structural phase transitions of superionic conductor CsHSO4. Ferroelectrics 1985, 63, 59–67. [Google Scholar] [CrossRef]
- Belushkin, A.V.; Adams, M.A.; Kolesnikov, A.I.; Shuvalov, L.A. Lattice dynamics and effects of anharmonicity in different phases of caesium hydrogen sulphate. J. Phys. Condens. Matter 1994, 6, 5823–5832. [Google Scholar] [CrossRef]
- Krzystyniak, M.; Druzbicki, K.; Fernandez-Alonso, F. Nuclear dynamics in the metastable phase of the solid acid caesium hydrogen sulfate. Phys. Chem. Chem. Phys. 2015, 46, 31287–31296. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.C.H.; Parker, S.F.; Ramirez-Cuesta, A.J.; Tomkinson, J. Vibrational Spectroscopy with Neutrons, with Applications in Chemistry, Biology, Materials Science and Catalysis; World Scientific: Singapore, 2005. [Google Scholar]
- Parker, S.F.; Lennon, D.; Albers, P.W. Vibrational spectroscopy with neutrons: A review of new directions. Appl. Spectrosc. 2011, 65, 1325–1341. [Google Scholar] [CrossRef]
- Parker, S.F.; Lennon, D. Applications of neutron scattering to heterogeneous catalysis. J. Phys. Conf. Ser. 2016, 746, 012066. [Google Scholar] [CrossRef] [Green Version]
- Tomkinson, J.; Parker, S.F.; Lennon, D. No evidence for Evans’ holes in the A, B, C vibrational structure of potassium dihydrogen arsenate. J. Chem. Phys. 2010, 133, 034508. [Google Scholar] [CrossRef]
- Parker, S.F. The role of hydroxyl groups in low temperature carbon monoxide oxidation. Chem. Commun. 2011, 47, 1988–1990. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Fernandez-Alonso, F.; Parker, S.F.; Cutler, D.J.; King, A. Simultaneous neutron scattering and Raman scattering. Appl. Spectrosc. 2009, 63, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Colognesi, D.; Celli, M.; Cilloco, F.; Newport, R.J.; Parker, S.F.; Rossi-Albertini, V.; Sacchetti, F.; Tomkinson, J.; Zoppi, M. TOSCA neutron spectrometer; the final configuration. Appl. Phys. A 2002, 74, S64–S66. [Google Scholar] [CrossRef] [Green Version]
- Albers, P.W.; Glenneberg, J.; Refson, K.; Parker, S.F. IINS study of the molecular properties of pure hydrogen peroxide and its water mixtures of different concentration. J. Chem. Phys. 2014, 140, 16450. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.K.; Callison, J.; Winfield, J.M.; Carr, R.H.; Eaglesham, A.; Sutherland, A.; Parker, S.F.; Lennon, D. Spectroscopic characterisation of model compounds, reactants and byproducts connected with an isocyanate production chain. Ind. Eng. Chem. Res. 2018, 57, 7355–7362. [Google Scholar] [CrossRef]
- Mazzei, L.; Perrichon, A.; Mancini, A.; Wahnström, G.; Malavasi, L.; Parker, S.F.; Börjesson, L.; Karlsson, M. Local structure and vibrational dynamics in indium-doped barium zirconate. J. Mater. Chem. A 2019, 7, 7360–7372. [Google Scholar] [CrossRef] [Green Version]
- ISIS Neutron and Muon Source. Available online: http://www.isis.stfc.ac.uk/Pages/sandals.aspx (accessed on 23 January 2020).
- ISIS Neutron and Muon Source. Available online: http://www.isis.stfc.ac.uk/ (accessed on 23 January 2020).
- Chisholm, C.R.I.; Haile, S.M. Entropy evaluation of the superprotonic phase of CsHSO4: Pauling’s ice rules adjusted for systems containing disordered hydrogen-bonded tetrahedra. Chem. Mater. 2007, 19, 270–279. [Google Scholar]
- Hannon, A.C.; Howells, W.S.; Soper, A.K. ATLAS: A suite of programs for the analysis of time-of-flight neutron diffraction data from liquid and amorphous samples. Inst. Phys. Conf. Ser. 1990, 107, 193–211. [Google Scholar]
- Farrow, C.L.; Juhás, P.; Liu, J.W.; Bryndin, D.; Božin, E.S.; Bloch, J.; Proffen, T.; Billinge, S.J.L. PDFfit2 and PDFgui: Computer programs for studying nanostructure in crystals. J. Phys. Condens. Matter 2007, 19, 335219. [Google Scholar] [CrossRef] [Green Version]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Krist.-Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Refson, K.; Tulip, P.R.; Clark, S.J. Variational density-functional perturbation theory for dielectrics and lattice dynamics. Phys. Rev. B 2006, 73, 155114. [Google Scholar] [CrossRef] [Green Version]
- Milman, V.; Perlov, A.; Refson, K.; Clark, S.J.; Gavartin, J.; Winkler, B. Structural, electronic and vibrational properties of tetragonal zirconia under pressure: A density functional theory study. J. Phys. Condens. Matter 2009, 21, 485404. [Google Scholar] [CrossRef] [PubMed]
- Refson, K. Phonons and Related Calculations in CASTEP. Available online: http://www.castep.org/ (accessed on 23 January 2020).
- Dassault Systèmes BIOVIA. Available online: https://www.3dsbiovia.com/products/collaborative-science/biovia-materials-studio/ (accessed on 23 January 2020).
- Ramirez-Cuesta, A.J. aCLIMAX 4.0.1, The new version of the software for analyzing and interpreting INS spectra. Comput. Phys. Commun. 2004, 157, 226–238. [Google Scholar] [CrossRef]
- Dymkowski, K.; Parker, S.F.; Fernandez-Alonso, F.; Mukhopadhyay, S. AbINS: The modern software for INS interpretation. Phys. B 2018, 551, 443–448. [Google Scholar] [CrossRef]









| Phase III | Phase II | Phase I | Assign 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cal 1 | INS 2 | Raman | Cal | INS | Raman | Cal | INS | Raman | |
| 10 K | 10 K | 10 K | 10 K | 430 K | 430 K | ||||
| 0–96 | 32–88 w | 0–96 | 25–1010 w | 0–93 | Translations | ||||
| 102 | 107 m | 103 | 105 w | 101 | HSO4 Libration | ||||
| 116 | 120 w | 123 | 123 w | 117 | HSO4 Libration | ||||
| 219 | 202,212,218 w,w,w | 221 w | 199 | 192,203 sh,w | 194 | HSO4 Libration | |||
| 399 | 398 | 404 | 414 s | ν2 O–S–O bend | |||||
| 441 | 421 s | 432 | 425,441,462 m,m,m | 415,430 s,s | 426 | 430 m | 432 sh | ν2 O–S–O bend | |
| 547 | 549 | 552 | ν4 O–S–O bend | ||||||
| 560 | 572,589 m,m | 570,589 m,s | 561 | 590 m | 579,587 m,s | 561 | ν4 O–S–O bend | ||
| 593 | 606,614 m,m | 605,619 m,m | 580 | 600,612 m,m | 601 s | 577 | 582 s | ν4 O–S–O bend | |
| 822 | 866 s | 821 | 863 s | 818 | 823 m,br | S–OH stretch | |||
| 942 | 865,902 vs,s | 913 | 830 vs | 904 | 746 s | S–O–H oop bend | |||
| 984 | 995 vs | 1006 | 1032 w | 1016 vs | 1011 | 1027 vs | ν1 S–O sym stretch | ||
| 1133 | 1178 s | 1146 | 1173 m | 1140 | 1177 w,br | ν3 S–O asym stretch | |||
| 1252 | 1255 m | 1215 | 1256 m | 1210 | 1210 w,br | ν3 S–O asym stretch | |||
| 1372 | 1368 s | 1320 | 1332 s | 1324 | 1295 s | S–OH ip bend | |||
| 2589 | 2733 | 2757 | 3185 | O–H stretch | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, S.F.; Cavaye, H.; Callear, S.K. Structure and Dynamics of the Superprotonic Conductor Caesium Hydrogen Sulfate, CsHSO4. Molecules 2020, 25, 1271. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061271
Parker SF, Cavaye H, Callear SK. Structure and Dynamics of the Superprotonic Conductor Caesium Hydrogen Sulfate, CsHSO4. Molecules. 2020; 25(6):1271. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061271
Chicago/Turabian StyleParker, Stewart F., Hamish Cavaye, and Samantha K. Callear. 2020. "Structure and Dynamics of the Superprotonic Conductor Caesium Hydrogen Sulfate, CsHSO4" Molecules 25, no. 6: 1271. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061271