A Novel Alternative in the Treatment of Detrusor Overactivity? In Vivo Activity of O-1602, the Newly Synthesized Agonist of GPR55 and GPR18 Cannabinoid Receptors
Abstract
:1. Introduction
2. Results
2.1. Cystometry
2.2. Diuresis and Cardiovascular Parameters
2.3. Biochemical Studies
3. Discussion
3.1. Cystometry
3.2. Diuresis and Cardiovascular Parameters
3.3. Biochemical Studies
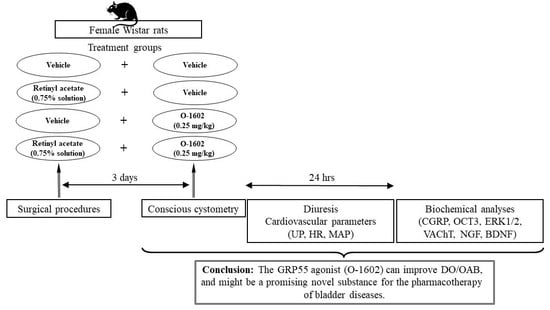
4. Materials and Methods
4.1. Experimental Animals
4.2. Drugs
4.3. Treatment Schedule
- 1st group received saline (the control group, CON)
- 2nd group received RA
- 3rd group received O-1602 (0.25 mg/kg)
- 4th group received RA and O-1602 (0.25 mg/kg)
4.4. Surgical Procedures
4.5. Cystometry
4.6. Diuresis and Cardiovascular Parameters
4.7. Biochemical Studies
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-HT | – serotoninergic receptor |
| AMPA | – α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| ANVC | – nonvoiding contractions amplitude (cmH2O) |
| AUC | – area under the pressure curve (cmH2O/sec) |
| BC | – bladder compliance (mL/cmH2O) |
| BCD | – bladder contraction duration (s) |
| BDNF | – brain-derived neurotrophic factor |
| BP | – basal pressure (cmH2O) |
| cAMP | – cyclic-adenosine monophosphate |
| CB | – cannabinoid receptor |
| CGRP | – calcitonin gene related peptide |
| DO | – detrusor overactivity |
| DOI | – detrusor overactivity index (cmH2O/mL) |
| ERK 1/2 | – extracellular signal-regulated kinase 1/2 |
| FNVC | – nonvoiding contractions frequency (times/filling phase) |
| GPR18 | – orphan G-protein–coupled cannabinoid receptor |
| GPR55 | – orphan metabotropic cannabinoid receptor |
| HR | – heart rate (beats/min) |
| ICI | – intercontraction interval (s) |
| LPI | – L-alpha-lysophosphatidylinositol |
| LUT | – lower urinary tract |
| MAP | – arterial pressure (mmHg) |
| MF | – micturition frequency |
| MVP | – micturition voiding pressure (cmH2O) |
| NGF | – nerve growth factor |
| O-1602 | – agonist of GPR55 and GPR18 cannabinoid receptor |
| OAB | – overactive bladder syndrome |
| OCT3 | – organic cation transporter 3 |
| PVR | – post-void residual (mL) |
| RA | – retinyl acetate |
| ROCK | – Rho-kinase |
| RT | – relaxation time (s) |
| TP | – threshold pressure (cmH2O) |
| TRPV1 | – transient receptor potential vanilloid 1 |
| TRPV4 | – transient receptor potential vanilloid 4 |
| UP | – urine production (mL/day) |
| VAChT | – vesicular acetylcholine transporter |
| VE | – voiding efficiency (%) |
| VT | – volume threshold (mL) |
| VTNVC | – volume threshold to elicit NVC (%) |
| VV | – voided volume (mL) |
References
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology of lower urinary tract function: Report from the standardisation sub-committee of the international continence society. Am. J. Obstet. Gynecol. 2002, 187, 116–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrams, P.; Artibani, W.; Cardozo, L.; Dmochowski, R.; Van Kerrebroeck, P.; Sand, P. Reviewing the ICS 2002 terminology report: The ongoing debate. Neurourol. Urodynamics 2009, 28, 287. [Google Scholar] [CrossRef] [PubMed]
- Gulur, D.M.; Drake, M.J. Management of overactive bladder. Nat. Rev. Urol. 2010, 7, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Wagg, A.; Compion, G.; Fahey, A.; Siddiqui, E. Persistence with prescribed antimuscarinic therapy for overactive bladder: A UK experience. BJU Int. 2012, 110, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Bang, W.; Choi, H.G. Analysis of the prevalence of and factors associated with overactive bladder in adult Korean women. PLoS ONE 2017, 12, e0185592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, D.E.; Abrams, P.; Milsom, I.; Kopp, Z.; Reilly, K.; EPIC Study group. Understanding the elements of overactive bladder: Questions raised by the EPIC study. BJU Int. 2008, 101, 1381–1387. [Google Scholar] [CrossRef]
- Stewart, W.F.; Van Rooyen, J.B.; Cundiff, G.W.; Abrams, P.; Herzog, A.R.; Corey, R.; Hunt, T.L.; Wein, A.J. Prevalence and burden of overactive bladder in the United States. World J. Urol. 2003, 20, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Patra, P.B.; Patra, S. Sex differences in the physiology and pharmacology of the lower urinary tract. Curr. Urol. 2013, 6, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Eapen, R.S.; Radomski, S. Review of the epidemiology of overactive bladder. Res. Rep. Urol. 2016, 8, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Link, C.L.; Steers, W.D.; Kusek, J.W.; McKinlay, J.B. The Association of Adiposity and Overactive Bladder Appears to Differ by Gender: Results from the Boston Area Community Health Survey. J. Urol. 2011, 185, 955–963. [Google Scholar] [CrossRef] [Green Version]
- Bauer, M.R.M.; Huebner, W. Gender differences in bladder control: From babies to elderly. World J. Urol. 2013, 31, 1081–1085. [Google Scholar] [CrossRef]
- Coyne, K.S.; Sexton, C.C.; Irwin, D.E.; Kopp, Z.; Kelleher, C.J.; Milsom, I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: Results from the EPIC study. BJU Int. 2008, 101, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.E.; Milsom, I.; Hunskaar, S.; Reilly, K.; Kopp, Z.; Herschorn, S.; Coyne, K.; Kelleher, C.; Hampel, C.; Artibani, W.; et al. Population-Based Survey of Urinary Incontinence, Overactive Bladder, and Other Lower Urinary Tract Symptoms in Five Countries: Results of the EPIC Study. Eur. Urol. 2006, 50, 1306–1315. [Google Scholar] [CrossRef]
- Irwin, D.E.; Milsom, I.; Kopp, Z.; Abrams, P. Symptom Bother and Health Care–Seeking Behavior among Individuals with Overactive Bladder. Eur. Urol. 2008, 53, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Wein, A.J. Recent advances in management of bladder overactivity. F1000 Med. Rep. 2010, 2, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.R. Muscarinic receptor antagonists in the treatment of overactive bladder. Urology 2000, 55, 33–46. [Google Scholar] [CrossRef]
- Nabi, G.; Cody, J.D.; Ellis, G.; Herbison, P.; Hay-Smith, J.; Herbison, G.P. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database Syst. Rev. 2006, 4, CD003781. [Google Scholar] [CrossRef]
- Rudy, D.; Cline, K.; Harris, R.; Goldberg, K.; Dmochowski, R. Time to onset of improvement in symptoms of overactive bladder using antimuscarinic treatment. BJU Int. 2006, 97, 540–546. [Google Scholar] [CrossRef]
- Peters, S.L.; Schmidt, M.; Michel, M.C. Rho kinase: A target for treating urinary bladder dysfunction? Trends Pharmacol. Sci. 2006, 27, 492–497. [Google Scholar] [CrossRef]
- Wróbel, A.; Doboszewska, U.; Rechberger, E.; Rojek, K.; Serefko, A.; Poleszak, E.; Skalicka-Woźniak, K.; Dudka, J.; Wlaź, P. Rho kinase inhibition ameliorates cyclophosphamide-induced cystitis in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Nicol, G.D. NGF-mediated sensitization of the excitability of rat sensory neurons is prevented by a blocking antibody to the p75 neurotrophin receptor. Neurosci. Lett. 2004, 366, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Doboszewska, U.; Rechberger, E.; Bańczerowska-Górska, M.; Czuczwar, P.; Poleszak, E.; Dudka, J.; Wlaź, P.; Miotła, P.; Wlaźlak, E.; et al. Blebbistatin, a Myosin II Inhibitor, Exerts Antidepressant-Like Activity and Suppresses Detrusor Overactivity in an Animal Model of Depression Coexisting with Overactive Bladder. Neurotox. Res. 2018, 35, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Shieh, C.C.; Feng, J.; Buckner, S.; Brioni, J.D.; Coghlan, M.J.; Sullivan, J.P.; Gopalakrishnan, M. Functional implication of spare ATP-sensitive K(+) channels in bladder smooth muscle cells. J. Pharmacol. Exp. Ther. 2001, 296, 669–675. [Google Scholar] [PubMed]
- Espey, M.J.; Downie, J.W. Serotonergic modulation of cat bladder function before and after spinal transection. Eur. J. Pharmacol. 1995, 287, 173–177. [Google Scholar] [CrossRef]
- Yoshimura, N.; De Groat, W.C. Neural Control of the Lower Urinary Tract. Int. J. Urol. 1997, 4, 111–125. [Google Scholar] [CrossRef] [PubMed]
- De Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 5, 327–396. [Google Scholar]
- Cerruto, M.; Asimakopoulos, A.D.; Artibani, W.; Del Popolo, G.; La Martina, M.; Carone, R.; Agrò, E.F. Insight into New Potential Targets for the Treatment of Overactive Bladder and Detrusor Overactivity. Urol. Int. 2012, 89, 1–8. [Google Scholar] [CrossRef]
- Ohlstein, E.H.; von Keitz, A.; Michel, M.C. A multicenter, double-blind, randomized, placebo-controlled trial of the beta3-adrenoceptor agonist solabegron for overactive bladder. Eur. Urol. 2012, 62, 834–840. [Google Scholar] [CrossRef]
- Digesu, G.A.; Khullar, V.; Bhide, A.; Fernando, R. Mirabegron – a selective ß3-adrenoreceptor agonist for the treatment of overactive bladder. Res. Rep. Urol. 2012, 4, 41–45. [Google Scholar]
- Wrobel, A.; Miziak, B.; Banczerowska-Gorska, M.; Szopa, A.; Serefko, A.; Stangel-Wojcikiewicz, K.; Czuczwar, P.; Laskowska, M.; Wlazlak, E.; Dudka, J.; et al. The influence of nebivolol on the activity of BRL 37344 - the beta3-adrenergic receptor agonist, in the animal model of detrusor overactivity. Neurourol. Urodyn. 2019, 38, 1229–1240. [Google Scholar] [CrossRef]
- Crescioli, C.; Morelli, A.; Adorini, L.; Ferruzzi, P.; Luconi, M.; Vannelli, G.B.; Marini, M.; Gelmini, S.; Fibbi, B.; Donati, S.; et al. Human Bladder as a Novel Target for Vitamin D Receptor Ligands. J. Clin. Endocrinol. Metab. 2005, 90, 962–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wróbel, A.; Rechberger, T. The Influence of Maxacalcitol, Vitamin D3 Analog, on Detrusor Overactivity in Conscious Rats. Urol. 2016, 93, 224.e7–224.e15. [Google Scholar] [CrossRef] [PubMed]
- Chopra, B.; Barrick, S.R.; Meyers, S.; Beckel, J.; Zeidel, M.L.; Ford, A.P.D.W.; De Groat, W.C.; Birder, L.A. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J. Physiol. 2004, 562, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, L.; Lose, G.; McClish, D.; Versi, E. A systematic review of the effects of estrogens for symptoms suggestive of overactive bladder. Acta Obstet. Gynecol. Scand. 2004, 83, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Lecci, A.; Giuliani, S.; Meini, S.; Maggi, C. Nociceptin and the micturition reflex. Peptides 2000, 21, 1007–1021. [Google Scholar] [CrossRef]
- Rechberger, T.; Nowakowski, Ł.; Rechberger, E.; Ziętek, A.; Winkler, I.; Miotła, P. Prevalence of common comorbidities among urogynaecological patients. Ginekol. Polska 2016, 87, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Ost, D.; Roskams, T.; Van Der Aa, F.; De, R.D. Topography of the vanilloid receptor in the human bladder: More than just the nerve fibers. J. Urol. 2002, 168, 293–297. [Google Scholar] [CrossRef]
- Merrill, L.; Vizzard, M.A. Intravesical TRPV4 blockade reduces repeated variate stress-induced bladder dysfunction by increasing bladder capacity and decreasing voiding frequency in male rats. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R471–R480. [Google Scholar] [CrossRef] [Green Version]
- Lecci, A.A.; Maggi, C. Tachykinins as modulators of the micturition reflex in the central and peripheral nervous system. Regul. Pept. 2001, 101, 1–18. [Google Scholar] [CrossRef]
- Yotsuyanagi, S.; Yokoyama, O.; Komatsu, K.; Kodama, K.; Niikura, S.; Namiki, M. Expression of neural plasticity related gene in the pontine tegmental area of rats with overactive bladder after cerebral infarction. J. Urol. 2001, 166, 1148–1155. [Google Scholar] [CrossRef] [Green Version]
- Igawa, Y.; Mattiasson, A.; Andersson, K.-E. Effects of GABA-Receptor Stimulation and Blockade on Micturition in Normal Rats and Rats with Bladder Outflow Obstruction. J. Urol. 1993, 150, 537–542. [Google Scholar] [CrossRef]
- Antonio, C.; Giovanni, P.; Conte, A.; Gino, B.; Elisa, I.; Chiara, M.B.; Antonio, P.; Maurizio, I. Gabapentin Treatment of Neurogenic Overactive Bladder. Clin. Neuropharmacol. 2006, 29, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Serefko, A.; Szopa, A.; Rojek, K.; Poleszak, E.; Skalicka-Woźniak, K.; Dudka, J. Inhibition of the CRF1 receptor influences the activity of antidepressant drugs in the forced swim test in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.; Philips, B.J.; Su, R.; Smaldone, M.C.; Erickson, V.L.; Chancellor, M.B.; Yoshimura, N.; Tyagi, P. Differential Expression of Functional Cannabinoid Receptors in Human Bladder Detrusor and Urothelium. J. Urol. 2009, 181, 1932–1938. [Google Scholar] [CrossRef]
- Strittmatter, F.; Gandaglia, G.; Benigni, F.; Bettiga, A.; Rigatti, P.; Montorsi, F.; Gratzke, C.; Stief, C.; Colciago, G.; Hedlund, P. Expression of Fatty Acid Amide Hydrolase (FAAH) in Human, Mouse, and Rat Urinary Bladder and Effects of FAAH Inhibition on Bladder Function in Awake Rats. Eur. Urol. 2012, 61, 98–106. [Google Scholar] [CrossRef]
- Brady, C.M.; Dasgupta, R.; Dalton, C.; Wiseman, O.J.; Berkley, K.J.; Fowler, C.J. An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Mult. Scler. J. 2004, 10, 425–433. [Google Scholar] [CrossRef]
- Consroe, P.; Musty, R.; Rein, J.; Tillery, W.; Pertwee, R. The Perceived Effects of Smoked Cannabis on Patients with Multiple Sclerosis. Eur. Neurol. 1997, 38, 44–48. [Google Scholar] [CrossRef]
- Bakali, E.; Elliott, R.A.; Taylor, A.; Willets, J.; Konje, J.; Tincello, U.G. Distribution and function of the endocannabinoid system in the rat and human bladder. Int. Urogynecology J. 2012, 24, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Bakali, E.; Elliott, R.A.; Taylor, A.; Lambert, D.; Willets, J.M.; Tincello, U.G. Human urothelial cell lines as potential models for studying cannabinoid and excitatory receptor interactions in the urinary bladder. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 581–589. [Google Scholar] [CrossRef]
- Freeman, R.M.; Adekanmi, O.; Waterfield, M.R.; Waterfield, A.E.; Wright, D.; Zajicek, J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: A multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int. Urogynecology J. 2006, 17, 636–641. [Google Scholar] [CrossRef]
- Kavia, R.; De Ridder, D.; Constantinescu, C.; Stott, C.; Fowler, C.J.; Constantinescu, C.S. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult. Scler. J. 2010, 16, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Begg, M.; Pacher, P.; Bátkai, S.; Osei-Hyiaman, D.; Offertáler, L.; Mo, F.M.; Liu, J.; Kunos, G. Evidence for novel cannabinoid receptors. Pharmacol. Ther. 2005, 106, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Sawzdargo, M.; Nguyen, T.; Lee, D.K.; Lynch, K.R.; Cheng, R.; Heng, H.H.; George, S.R.; O’Dowd, B.F. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Mol. Brain Res. 1999, 64, 193–198. [Google Scholar] [CrossRef]
- Mackie, K.; Stella, N. Cannabinoid Receptors and Endocannabinoids: Evidence for New Players. AAPS J. 2006, 8, E298. [Google Scholar] [CrossRef]
- Gratzke, C.; Streng, T.; Park, A.; Christ, G.; Stief, C.G.; Hedlund, P.; Andersson, K.-E. Distribution and Function of Cannabinoid Receptors 1 and 2 in the Rat, Monkey and Human Bladder. J. Urol. 2009, 181, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Bakali, E.; Tincello, D.G. Cannabinoids and the Urinary Bladder. Gynecol. Obstet. 2013, 3, 163. [Google Scholar] [CrossRef] [Green Version]
- Alavi, M.S.; Shamsizadeh, A.; Azhdari-Zarmehri, H.; Roohbakhsh, A. Orphan G protein-coupled receptors: The role in CNS disorders. Biomed. Pharmacother. 2018, 98, 222–232. [Google Scholar] [CrossRef]
- Sharir, H.; Abood, M. Pharmacological characterization of GPR55, a putative cannabinoid receptor. Pharmacol. Ther. 2010, 126, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Gratzke, C.; Weinhold, P.; Selwood, D.; Stief, C.G.; Andersson, K.; Hedlund, P. Distribution of the G-protein-coupled receptor 55 (GPR55) in the rat bladder and urodynamic effects of a selective GPR55-agonist in conscious rats. Eur. Urol. Suppl. 2009, 8, 271. [Google Scholar] [CrossRef]
- Baker, D.; Pryce, G.; Davies, W.; Hiley, C.R. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol. Sci. 2006, 27, 1–4. [Google Scholar] [CrossRef]
- Ashton, J.C. The atypical cannabinoid O-1602: Targets, actions, and the central nervous system. Central Nerv. Syst. Agents Med. Chem. 2012, 12, 233–239. [Google Scholar] [CrossRef]
- Console-Bram, L.; Brailoiu, E.; Brailoiu, G.C.; Sharir, H.; Abood, M. Activation of GPR18 by cannabinoid compounds: A tale of biased agonism. Br. J. Pharmacol. 2014, 171, 3908–3917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wróbel, A.; Łańcut, M.; Rechberger, T. A new model of detrusor overactivity in conscious rats induced by retinyl acetate instillation. J. Pharmacol. Toxicol. Methods 2015, 74, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Nowakowski, Ł.; Doboszewska, U.; Rechberger, E.; Bańczerowska-Górska, M.; Wlaźlak, E.; Zakrocka, I.; Wlaź, P.; Semczuk, A.; Dudka, J.; et al. Blebbistatin reveals beneficial effects on the cystometric parameters in an animal model of detrusor overactivity. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayn, M.H.; Ballesteros, I.; De Miguel, F.; Coyle, C.H.; Tyagi, S.; Yoshimura, N.; Chancellor, M.B.; Tyagi, P. Functional and Immunohistochemical Characterization of CB1 and CB2 Receptors in Rat Bladder. Urology 2008, 72, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Merriam, F.V.; Wang, Z.-Y.; Guerios, S.D.; Bjorling, D. Cannabinoid receptor 2 is increased in acutely and chronically inflamed bladder of rats. Neurosci. Lett. 2008, 445, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Walczak, J.; Price, T.J.; Cervero, F. Cannabinoid CB1 receptors are expressed in the mouse urinary bladder and their activation modulates afferent bladder activity. Neuroscience 2009, 159, 1154–1163. [Google Scholar] [CrossRef]
- Gratzke, C.; Streng, T.; Stief, C.G.; Downs, T.R.; Alroy, I.; Rosenbaum, J.S.; Andersson, K.; Hedlund, P. Effects of Cannabinor, a Novel Selective Cannabinoid 2 Receptor Agonist, on Bladder Function in Normal Rats. Eur. Urol. 2010, 57, 1093–1100. [Google Scholar] [CrossRef]
- Mukerji, G.; Yiangou, Y.; Agarwal, S.K.; Anand, P. Increased Cannabinoid Receptor 1-Immunoreactive Nerve Fibers in Overactive and Painful Bladder Disorders and Their Correlation with Symptoms. Urology 2010, 75, 1514.e15–1514.e20. [Google Scholar] [CrossRef]
- Walczak, J.; Cervero, F. Local activation of cannabinoid CB1 receptors in the urinary bladder reduces the inflammation-induced sensitization of bladder afferents. Mol. Pain 2011, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Gratzke, C.; Streng, T.; Stief, C.G.; Alroy, I.; Limberg, B.J.; Downs, T.R.; Rosenbaum, J.S.; Hedlund, P.; Andersson, K.-E. Cannabinor, a Selective Cannabinoid-2 Receptor Agonist, Improves Bladder Emptying in Rats With Partial Urethral Obstruction. J. Urol. 2011, 185, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, P. Cannabinoids and the endocannabinoid system in lower urinary tract function and dysfunction. Neurourol. Urodynamics 2013, 33, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Strittmatter, F.; La Croce, G.; Benigni, F.; Bettiga, A.; Castiglione, F.; Moschini, M.; Montorsi, F.; Gratzke, C.; Montorsi, F.; et al. The fatty acid amide hydrolase inhibitor oleoyl ethyl amide counteracts bladder overactivity in female rats. Neurourol. Urodynamics 2013, 33, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, A.; Rechberger, T. The effect of combined treatment with a beta3 AR agonist and a ROCK inhibitor on detrusor overactivity. Neurourol. Urodyn. 2017, 36, 580–588. [Google Scholar] [CrossRef]
- Takeda, H.; Yamazaki, Y.; Akahane, M.; Igawa, Y.; Ajisawa, Y.; Nishizawa, O. Role of the beta(3)-adrenoceptor in urine storage in the rat: Comparison between the selective beta(3)-adrenoceptor agonist, CL316, 243, and various smooth muscle relaxants. J. Pharmacol. Exp. Ther. 2000, 293, 939–945. [Google Scholar]
- Cannon, T.W.; Damaser, M.S. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci. 2001, 69, 1193–1202. [Google Scholar]
- Abrams, P.; Andersson, K.-E. Muscarinic receptor antagonists for overactive bladder. BJU Int. 2007, 100, 987–1006. [Google Scholar] [CrossRef]
- Miller, K.L.; DuBeau, C.E.; Bergmann, M.; Griffiths, D.J.; Resnick, N.M. Quest for a detrusor overactivity index. J. Urol. 2002, 167, 578–584. [Google Scholar] [CrossRef]
- Abrams, P. Describing bladder storage function: Overactive bladder syndrome and detrusor overactivity. Urology 2003, 62, 28–37. [Google Scholar] [CrossRef]
- Juszczak, K.; Ziomber, A.; Wyczółkowski, M.; Thor, P.J. Hyperosmolarity alters micturition: A comparison of urinary bladder motor activity in hyperosmolar and cyclophosphamide-induced models of overactive bladder. Can. J. Physiol. Pharmacol. 2010, 88, 899–906. [Google Scholar] [CrossRef]
- Cruz, F.J.; Herschorn, S.; Aliotta, P.; Brin, M.; Thompson, C.; Lam, W.; Daniell, G.; Heesakkers, J.P.F.A.; Haag-Molkenteller, C. Efficacy and Safety of OnabotulinumtoxinA in Patients with Urinary Incontinence Due to Neurogenic Detrusor Overactivity: A Randomised, Double-Blind, Placebo-Controlled Trial. Eur. Urol. 2011, 60, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Behr-Roussel, D.; Oger, S.; Pignol, B.; Pham, E.; Le Maux, A.; Chabrier, P.-E.; Caisey, S.; Compagnie, S.; Picaut, P.; Bernabé, J.; et al. Minimal Effective Dose of Dysport and Botox in a Rat Model of Neurogenic Detrusor Overactivity. Eur. Urol. 2012, 61, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, P.; Gratzke, C.; Streng, T.; Stief, C.; Andersson, K.-E.; Hedlund, P. TRPA1 Receptor Induced Relaxation of the Human Urethra Involves TRPV1 and Cannabinoid Receptor Mediated Signals, and Cyclooxygenase Activation. J. Urol. 2010, 183, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.J.; Ross, R.; Whyte, J.; Henstridge, C.; Irving, A.; McGrath, J. Fluorescent ligand binding reveals heterogeneous distribution of adrenoceptors and ‘cannabinoid-like’ receptors in small arteries. Br. J. Pharmacol. 2010, 159, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Fukao, M.; Hattori, Y.; Kanno, M.; Sakuma, I.; Kitabatake, A. Structural Differences in the Ability of Lysophospholipids to Inhibit Endothelium-Dependent Hyperpolarization by Acetylcholine in Rat Mesenteric Arteries. Biochem. Biophys. Res. Commun. 1996, 227, 479–483. [Google Scholar] [CrossRef]
- AlSuleimani, Y.M.; Hiley, C. The GPR55 agonist lysophosphatidylinositol relaxes rat mesenteric resistance artery and induces Ca2+ release in rat mesenteric artery endothelial cells. Br. J. Pharmacol. 2015, 172, 3043–3057. [Google Scholar] [CrossRef] [Green Version]
- Karpińska, O.; Baranowska-Kuczko, M.; Malinowska, B.; Kloza, M.; Kusaczuk, M.; Gęgotek, A.; Golec, P.; Kasacka, I.; Kozłowska, H. Mechanisms of l-alpha-lysophosphatidylinositol-induced relaxation in human pulmonary arteries. Life Sci. 2018, 192, 38–45. [Google Scholar] [CrossRef]
- Yu, J.; Deliu, E.; Zhang, X.-Q.; Hoffman, N.E.; Carter, R.L.; Grisanti, L.A.; Brailoiu, G.C.; Madesh, M.; Cheung, J.Y.; Force, T.; et al. Differential Activation of Cultured Neonatal Cardiomyocytes by Plasmalemmal Versus Intracellular G Protein-coupled Receptor 55*. J. Boil. Chem. 2013, 288, 22481–22492. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.; Kwon, B.-E. Overactive Bladder is a Distress Symptom in Heart Failure. Int. Neurourol. J. 2018, 22, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Andersson, K.; Sarawate, C.; Kahler, K.H.; Stanley, E.L.; Kulkarni, A.S. Cardiovascular morbidity, heart rates and use of antimuscarinics in patients with overactive bladder. BJU Int. 2009, 106, 268–274. [Google Scholar] [CrossRef]
- Chiu, A.-F.; Liao, C.-H.; Wang, C.-C.; Wang, J.-H.; Tsai, C.-H.; Kuo, H. High Classification of Chronic Heart Failure Increases Risk of Overactive Bladder Syndrome and Lower Urinary Tract Symptoms. Urology 2012, 79, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.H.; Hardin, S.R.; Behrend, C.; Collins, S.K.-R.; Madigan, C.K.; Carlson, J.R. Urinary Incontinence and Overactive Bladder in Patients With Heart Failure. J. Urol. 2009, 182, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.M.; Baccino, D.; Valbusa, A.; Scala, C.; Barra, F.; Brunelli, C.; Ferrero, S. Cardiovascular effects of antimuscarinic agents and beta3-adrenergic receptor agonist for the treatment of overactive bladder. Expert Opin. Drug Saf. 2018, 17, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Hald, T.; Barnard, R.J.; Holm-Bentzen, M. Treatment of Interstitial Cystitis. Sens. Disord. Bladder Urethra 1986, 63, 73–78. [Google Scholar]
- Peyronnet, B.; Bendavid, C.; Manunta, A.; Damphousse, M.; Cheensse, C.; Brochard, C.; Castel-Lacanal, E.; Siproudhis, L.; Bensalah, K.; Game, X. [The role of urinary markers in the assessment and follow-up of lower urinary tract disorders: A literature review]. Prog. Urol. 2015, 25, 188–199. [Google Scholar] [CrossRef]
- Pennycuff, J.F.; Schutte, S.; Hudson, C.O.; Karp, D.R.; Malykhina, A.P.; Northington, G.M. Urinary neurotrophic peptides in postmenopausal women with and without overactive bladder. Neurourol. Urodynamics 2016, 36, 740–744. [Google Scholar] [CrossRef]
- Tian, X.-J.; Liu, C.; Liu, K.; Tang, S.-Y. Urinary biomarkers of overactive bladder. Chin. Med. J. 2019, 132, 1104–1106. [Google Scholar] [CrossRef] [Green Version]
- Suh, Y.S.; Ko, K.J.; Kim, T.H.; Lee, H.S.; Lee, K.-S.; Cho, W.J.; Lee, M.; Lee, K.-S. Potential Biomarkers for Diagnosis of Overactive Bladder Patients: Urinary Nerve Growth Factor, Prostaglandin E2, and Adenosine Triphosphate. Int. Neurourol. J. 2017, 21, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Vizzard, M.A. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J. Chem. Neuroanat. 2001, 21, 125–138. [Google Scholar] [CrossRef]
- Ellington, H.C.; Cotter, M.; Cameron, N.; Ross, R.A. The effect of cannabinoids on capsaicin-evoked calcitonin gene-related peptide (CGRP) release from the isolated paw skin of diabetic and non-diabetic rats. Neuropharmacology 2002, 42, 966–975. [Google Scholar] [CrossRef]
- Tyagi, P.; Tyagi, V.; Yoshimura, N.; Chancellor, M. Functional role of cannabinoid receptors in urinary bladder. Indian J. Urol. 2010, 26, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Hanna-Mitchell, A.T.; Beckel, J.; Barbadora, S.; Kanai, A.J.; De Groat, W.C.; Birder, L.A. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 2007, 80, 2298–2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessler, I.; Rõth, E.; Deutsch, C.; Brockerhoff, P.; Bittinger, F.; Kirkpatrick, C.J.; Kilbinger, H. Release of non-neuronal acetylcholine from the isolated human placenta is mediated by organic cation transporters. Br. J. Pharmacol. 2001, 134, 951–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lips, K.S.; Volk, C.; Schmitt, B.M.; Pfeil, U.; Arndt, P.; Miska, D.; Ermert, L.; Kummer, W.; Koepsell, H. Polyspecific Cation Transporters Mediate Luminal Release of Acetylcholine from Bronchial Epithelium. Am. J. Respir. Cell Mol. Boil. 2005, 33, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koepsell, H.; Schmitt, B.M.; Gorboulev, V. Organic cation transporters. Rev. Physiol. Biochem. Pharmacol. 2003, 150, 36–90. [Google Scholar] [PubMed]
- Hegde, S.S. Muscarinic receptors in the bladder: From basic research to therapeutics. Br. J. Pharmacol. 2006, 147, S80–S87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckel, J.; Kanai, A.; Lee, S.-J.; De Groat, W.C.; Birder, L.A. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am. J. Physiol. Physiol. 2005, 290, F103–F110. [Google Scholar] [CrossRef] [Green Version]
- Andersson, K.; Yoshida, M. Antimuscarinics and the Overactive Detrusor—Which Is the Main Mechanism of Action? Eur. Urol. 2003, 43, 1–5. [Google Scholar] [CrossRef]
- Ji, R.-R. Mitogen-activated protein kinases as potential targets for pain killers. Curr. Opin. Investig. Drugs 2004, 5, 71–75. [Google Scholar]
- Cruz, C.D.; Avelino, A.; McMahon, S.B.; Cruz, F.J. Increased spinal cord phosphorylation of extracellular signal-regulated kinases mediates micturition overactivity in rats with chronic bladder inflammation. Eur. J. Neurosci. 2005, 21, 773–781. [Google Scholar] [CrossRef]
- Marentette, J.O.; Hauser, P.J.; Hurst, R.; Klumpp, D.J.; Rickard, A.; McHowat, J. Tryptase Activation of Immortalized Human Urothelial Cell Mitogen-Activated Protein Kinase. PLoS ONE 2013, 8, e69948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alique, M.; Lucio, F.J.; Herrero, J.F. Vitamin A active metabolite, all-trans retinoic acid, induces spinal cord sensitization. II. Effects after intrathecal administration. Br. J. Pharmacol. 2006, 149, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Pezet, S.; McMahon, S.B. NEUROTROPHINS: Mediators and Modulators of Pain. Annu. Rev. Neurosci. 2006, 29, 507–538. [Google Scholar] [CrossRef] [PubMed]
- Ochodnický, P.; Cruz, C.D.; Yoshimura, N.; Cruz, F.J. Neurotrophins as regulators of urinary bladder function. Nat. Rev. Urol. 2012, 9, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Kuo, H.C. Urinary Nerve Growth Factor Level Could be a Potential Biomarker for Diagnosis of Overactive Bladder. J. Urol. 2008, 179, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Steers, W.D.; Tuttle, J.B. Mechanisms of Disease: The role of nerve growth factor in the pathophysiology of bladder disorders. Nat. Clin. Pr. Urol. 2006, 3, 101–110. [Google Scholar] [CrossRef]
- Antunes-Lopes, T.; Cruz, C.D.; Cruz, F.J.; Sievert, K.D. Biomarkers in lower urinary tract symptoms/overactive bladder. Curr. Opin. Urol. 2014, 24, 352–357. [Google Scholar] [CrossRef]
- Antunes-Lopes, T.; Pinto, R.A.; Barros, S.C.; Botelho, F.; Silva, C.; Cruz, C.D.; Cruz, F.J. Urinary Neurotrophic Factors in Healthy Individuals and Patients with Overactive Bladder. J. Urol. 2013, 189, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Park, E.Y.; Seo, S.I.; Park, Y.H.; Hwang, T.-K. Nerve Growth Factor and Prostaglandins in the Urine of Female Patients With Overactive Bladder. J. Urol. 2006, 175, 1773–1776. [Google Scholar] [CrossRef]
- Alkis, O.; Zumrutbas, A.E.; Toktas, C.; Aybek, H.; Aybek, Z. The use of biomarkers in the diagnosis and treatment of overactive bladder: Can we predict the patients who will be resistant to treatment? Neurourol. Urodynamics 2015, 36, 390–393. [Google Scholar] [CrossRef]
- Rachaneni, S.; Arya, P.; Latthe, P. Urinary nerve growth factor: A biomarker of detrusor overactivity? A systematic review. Int. Urogynecology J. 2013, 24, 1603–1609. [Google Scholar] [CrossRef]
- Antunes-Lopes, T.; Cruz, F. Urinary Biomarkers in Overactive Bladder: Revisiting the Evidence in 2019. Eur. Urol. Focus 2019, 5, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Chancellor, M.B.; Kuo, H.C. Decrease of urinary nerve growth factor levels after antimuscarinic therapy in patients with overactive bladder. BJU. Int. 2009, 103, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-T.; Lin, H.; Kuo, H.-C. Increased serum nerve growth factor levels in patients with overactive bladder syndrome refractory to antimuscarinic therapy. Neurourol. Urodynamics 2011, 30, 1525–1529. [Google Scholar] [CrossRef]
- Liu, H.-T.; Chancellor, M.B.; Kuo, H.-C. Urinary Nerve Growth Factor Levels are Elevated in Patients with Detrusor Overactivity and Decreased in Responders to Detrusor Botulinum Toxin-A Injection. Eur. Urol. 2009, 56, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.A.; Frias, B.; Allen, S.; Dawbarn, D.; McMahon, S.B.; Cruz, F.J.; Cruz, C.D. Sequestration of brain derived nerve factor by intravenous delivery of TrkB-Ig2 reduces bladder overactivity and noxious input in animals with chronic cystitis. Neuroscience 2010, 166, 907–916. [Google Scholar] [CrossRef]
- Frias, B.; Allen, S.; Dawbarn, D.; Charrua, A.; Cruz, F.J.; Cruz, C.D. Brain-derived neurotrophic factor, acting at the spinal cord level, participates in bladder hyperactivity and referred pain during chronic bladder inflammation. Neuroscience 2013, 234, 88–102. [Google Scholar] [CrossRef]
- Jaggar, S.; Hasnie, F.S.; Sellaturay, S.; Rice, A.P. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain 1998, 76, 189–199. [Google Scholar] [CrossRef]
- Farquhar-Smith, W.P.; Rice, A. Administration of endocannabinoids prevents a referred hyperalgesia associated with inflammation of the urinary bladder. Anesthesiology 2001, 94, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Farquhar-Smith, P.W.; Jaggar, S.I.; Rice, A.S.; Farquhar-Smith, W. Attenuation of nerve growth factor-induced visceral hyperalgesia via cannabinoid CB1 and CB2-like receptors. Pain 2002, 97, 11–21. [Google Scholar] [CrossRef]
- Farquhar-Smith, W.P.; Rice, A.S.C. A novel neuroimmune mechanism in cannabinoid-mediated attenuation of nerve growth factor-induced hyperalgesia. Anesthesiology 2003, 99, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Rechberger, T. The influence of Rho-kinase inhibition on acetic acid-induced detrusor overactivity. Neurourol. Urodynamics 2015, 36, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Serefko, A.; Bańczerowska-Górska, M.; Szopa, A.; Dudka, J.; Poleszak, E. Intravesical administration of blebbistatin prevents cyclophosphamide-induced toxicity of the urinary bladder in female Wistar rats. Neurourol. Urodynamics 2019, 38, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| Cystometric Parameters | CON | RA | O-1602 | RA + O-1602 | |||
|---|---|---|---|---|---|---|---|
| ANVC [cmH2O] | 2.221 ± 0.053 | 4.870± 0.442 | **** ↑ ×××× ↑ | 2.207 ± 0.064 | ^^^^ ↓ | 3.027 ± 0.203 | ^^^^ ↓ |
| AUC [cmH2O/sec] | 13.60 ± 0.486 | 18.67± 0.785 | **** ↑ ×× ↑ | 11.33 ± 0.522 | ^^^^ ↓ ×××× ↓ | 15.40 ± 0.600 | ^^ ↓ |
| BC [ml/cmH2O] | 0.2007 ± 0.006 | 0.1503± 0.006 | **** ↓ ×××× ↓ | 0.2107 ± 0.009 | ^^^^ ↑ | 0.1948 ± 0.005 | ^^^^ ↑ |
| BCD [sec] | 27.12± 1.015 | 26.18± 1.186 | ns | 27.24 ± 1.126 | ns | 30.58 ± 1.847 | ns |
| BP [cmH2O] | 2.667± 0.211 | 4.547± 0.243 | **** ↑ ×××× ↑ | 2.373 ± 0.124 | ^^^^ ↓ | 3.033 ± 0.215 | ^^^^ ↓ |
| DOI [ml/cmH2O] | 71.33± 5.275 | 177.5± 10.24 | **** ↑ ×××× ↑ | 63.20 ± 5.183 | ^^^^ ↓ ×× ↓ | 100.5 ± 5.788 | * ↑ ^^^^ ↓ |
| FNVC [times/filling phase] | 0.5067 ± 0.056 | 5.855 ± 0.484 | **** ↑ ×××× ↑ | 0.2700 ± 0.038 | ^^^^ ↓ | 1.258 ± 0.240 | ^^^^ ↓ |
| ICI [sec] | 932.5 ± 31.87 | 647.6 ± 32.57 | **** ↓ ×××× ↓ | 860.9 ± 38.33 | ^^ ↑ | 912.4 ± 50.08 | ^^^^ ↑ |
| MVP [cmH2O] | 36.93 ± 2.281 | 32.51 ± 1.945 | ns | 39.85 ± 2.258 | ns | 33.66 ± 2.270 | ns |
| PVR [ml] | 0.0713 ± 0.006 | 0.0687 ± 0.005 | ns | 0.0614 ± 0.006 | ns | 0.0748 ± 0.004 | ns |
| RT [sec] | 18.66 ± 0.625 | 19.97 ± 0.778 | ns | 21.17 ± 0.646 | ns | 20.49 ± 0.910 | ns |
| TP [cmH2O] | 7.040 ± 0.309 | 4.960 ± 0.291 | *** ↓ ×××× ↓ | 6.653 ± 0.270 | ^^ ↑ | 7.507 ± 0.446 | ^^^^ ↑ |
| VE [%] | 88.13 ± 2.019 | 89.53 ± 1.978 | ns | 89.07 ± 2.050 | ns | 92.00 ± 1.100 | ns |
| VT [ml] | 0.7460 ± 0.036 | 0.4953 ± 0.026 | ** ↓ ×××× ↓ | 0.8740 ± 0.034 | ^^^^ ↑ | 0.8233 ± 0.070 | ^^^^ ↑ |
| VTNVC [%] | 63.11 ± 2.845 | 29.93 ± 1.359 | **** ↓ ×××× ↓ | 68.67 ± 4.527 | ^^^^ ↑ | 58.10 ± 3.978 | ^^^^ ↑ |
| VV [ml] | 0.9443 ± 0.046 | 0.5237 ± 0.035 | *** ↓ ×× ↓ | 0.7754 ± 0.068 | ^ ↑ | 0.8706 ± 0.098 | ^^ ↑ |
| Diuresis and Cardiovascular Parameters | CON | RA | O-1602 | RA + O-1602 | |||
|---|---|---|---|---|---|---|---|
| UP [mL/day] | 18.61 ± 0.52 | 17.69 ± 0.76 | ns | 20.07 ± 0.70 | ^ ↑ ×× ↑ | 16.99 ± 0.51 | ns |
| HR [beats/min] | 307.7 ± 8.574 | 318.8 ± 8.705 | ns | 295.5 ± 11.00 | ns | 290.9 ± 8.264 | ns |
| MAP [mmHg] | 104.2 ± 4.189 | 94.53 ± 2.888 | × ↑ | 110.4 ± 3.399 | ^ ↑ ×× ↑ | 91.27 ± 3.939 | ns |
| Biochemical Parameters | CON | RA | O-1602 | RA + O-1602 | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|
| In the bladder urothelium | CGRP [pg/mL] | 80.47 ± 4.967 | 324.3 ± 20.18 | **** ↑ ×××× ↑ | 75.07 ± 5.330 | ^^^^ ↓ | 91.60 ± 5.715 | ^^^^ ↓ | 🞍 CGRP is one of the bladder’s afferent activity markers 🞍 in DO the CGRP level ↑ |
| ERK1/2 [pg/mL] | 31035 ± 1423 | 99125 ± 3118 | **** ↑ ×××× ↑ | 26154 ± 2139 | ^^^^ ↓ ×××× ↓ | 69255 ± 4275 | **** ↑ ^^^^ ↓ | 🞍 ERK 1/2 plays an important role in the control of detrusor muscle tone and bladder reflex activity 🞍 in DO induced by pain stimuli the ERK 1/2 level ↑ | |
| OCT3 [pg/mL] | 971.7 ± 46.26 | 3640 ± 201.8 | **** ↑ ×××× ↑ | 870.1 ± 47.27 | ^^^^ ↓ | 1166 ± 61.68 | ^^^^ ↓ | 🞍 OTC3 is an organic cation transporter involved in the release of acetylcholine from non-neuronal cells, it occurs only in the bladder urothelium 🞍 in DO the OTC3 level ↑ | |
| In the bladder detrusor muscle | VAChT [pg/mL] | 3133 ± 186.5 | 16220 ± 744.9 | **** ↑ ×××× ↑ | 3947 ± 272.6 | ^^^^ ↓ ×××× ↓ | 7726 ± 425.3 | **** ↑ ^^^^ ↓ | 🞍 VAChT is a vesicular acetylcholine transporter that releases it from non-neuronal cells, it occurs only in detrusor cells 🞍 in DO the VAChT level ↑ |
| In the urine | BDNF [pg/mL] | 62.20 ± 2.536 | 166.5 ± 4.685 | **** ↑ ×××× ↑ | 59.00 ± 3.457 | ^^^^ ↓ | 70.60 ± 3.549 | ^^^^ ↓ | 🞍 BDNF and NGF belong to neurotrophins and are produced in the bladder’s urothelial and smooth muscle cells 🞍 in DO the BDNF and NGF level ↑ |
| NGF [pg/mL] | 46.07 ± 2.292 | 122.6 ± 5.058 | **** ↑ ×××× ↑ | 36.00 ± 1.555 | ^^^^ ↓ × ↓ | 51.53 ± 3.553 | ^^^^ ↓ | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel, A.; Szopa, A.; Serefko, A.; Poleszak, E. A Novel Alternative in the Treatment of Detrusor Overactivity? In Vivo Activity of O-1602, the Newly Synthesized Agonist of GPR55 and GPR18 Cannabinoid Receptors. Molecules 2020, 25, 1384. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061384
Wróbel A, Szopa A, Serefko A, Poleszak E. A Novel Alternative in the Treatment of Detrusor Overactivity? In Vivo Activity of O-1602, the Newly Synthesized Agonist of GPR55 and GPR18 Cannabinoid Receptors. Molecules. 2020; 25(6):1384. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061384
Chicago/Turabian StyleWróbel, Andrzej, Aleksandra Szopa, Anna Serefko, and Ewa Poleszak. 2020. "A Novel Alternative in the Treatment of Detrusor Overactivity? In Vivo Activity of O-1602, the Newly Synthesized Agonist of GPR55 and GPR18 Cannabinoid Receptors" Molecules 25, no. 6: 1384. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25061384