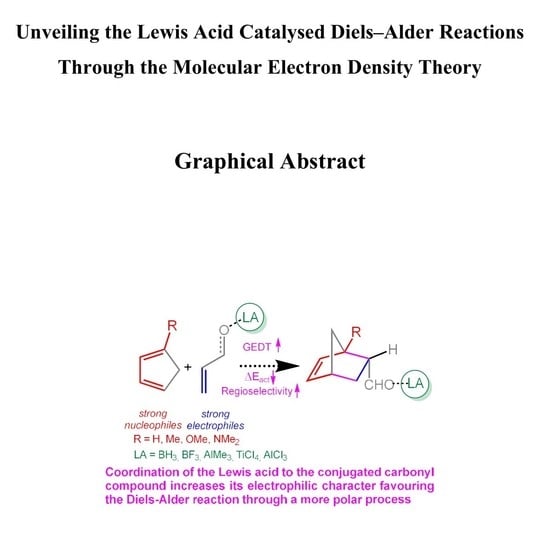
The present MEDT study has been divided in eight sections: i) in
Section 2.1, an analysis of the global and local CDFT reactivity indices at the ground state (GS) of the reagents is performed; ii) in
Section 2.2, an analysis of the effects of the LAs and the substitution on Cp
1 in the electronic structure of the reagents is carried out; iii) in
Section 2.3, the P-DA reactions between Cp
1 and the series of LA complexes are studied; iv) in
Section 2.4, P-DA reactions between substituted Cps
12 -
14 and complex
7-BF3 are studied; v) in
Section 2.5, the solvent effects in P-DA reactions between Cp
13 and complex
7-BF3 are evaluated; vi) in
Section 2.6, the thermodynamic parameters of the P-DA reactions between Cp
13 and complex
7-BF3 are discussed; vii) in
Section 2.7, the bonding changes along the P-DA reaction between Cp
1 and complex
7-BF3 are analyzed; and finally, and viii) in
Section 2.8, a comparative electron localization function (ELF) topological analysis between
two-stage one-step and two-step mechanisms in LA catalyzed DA reactions is carried out.
2.1. Analysis of the Global and Local CDFT Reactivity Indices at the GS of the Reagents
First, in order to understand the participation of the dienes and the LA complexes in these P-DA reactions, an analysis of the CDFT indices at the GS of the reagents, computed at the B3LYP/6-31G(d) level, was performed [
18]. The global CDFT indices, namely, electronic chemical potential μ, chemical hardness η, global electrophilicity ω, and global nucleophilicity
N, of the reagents are gathered in
Table 1.
The electronic chemical potential [
28,
29] μ of Cp
1 and the Cp derivatives
12–
14, between −3.01 (
1) and −2.09 (
14) eV, are higher than those of acrolein
7, μ = −4.38 eV, and the LA complexes, −4.61 (
7-AlMe3) and −6.20 (
7-BF3) eV, indicating that the GEDT in these P-DA reactions will take place from these Cp derivatives towards acrolein
7 and these LA complexes. Thus, these reactions are classified as the FEDF.
The electrophilicity [
15] ω and nucleophilicity [
16]
N indices of ethylene
5 are 0.73 and 1.87 eV, respectively, being classified as marginal electrophile and marginal nucleophile within the electrophilicity [
30] and nucleophilicity [
31] scales. These behaviours make that ethylene
5 does not participate in P-DA reaction.
The electrophilicity ω and nucleophilicity N indices of acrolein 7 are 1.84 and 2.13 eV, being classified as strong electrophile and moderate nucleophile. Inclusion of the electron-withdrawing (EW) carbonyl –CHO group in ethylene 5 markedly increases the electrophilicity of acrolein 7, allowing its participation in P-DA reactions as an electrophilic ethylene. A more remarkable effect is observed with the coordination of a LA to the oxygen atom of acrolein 7 and the electrophilicity ω index of these LA complexes rages from 3.20 (7-BH3) to 4.61 (7-AlCl3) eV. A clear correlation between the LA character of the LA salts and the increase of the electrophilic character of the corresponding LA complexes can be established. All these complexes have electrophilicity ω indices higher than 3.0 eV, accounting for the strong activation of acrolein 7. In general, the electrophilicity ω index of these LA complexes depends on the metal, increasing in the order B < Ti < Al. On the other hand, the counter ion of the LA salt has also a relevant role; thus, the electrophilicity ω index of 7-BF3, 3.29 eV, and 7-AlCl3, 4.61 eV, are higher than that of 7-BH3, 3.20 eV, and 7-AlMe3, 3.60 eV, respectively.
In general, a decrease of the nucleophilicity N indices of these LA complexes with the increase of its electrophilic character is observed. They have nucleophilicity N indices lower than 2.0, being classified as marginal nucleophiles. An unexpected value is found at of 7-AlMe3, N = 3.05 eV, which is classified as a strong nucleophile.
The electrophilicity ω and nucleophilicity
N indices of Cp
1 are 0.83 and 3.37 eV, respectively, being classified in the borderline of moderate electrophiles and as strong nucleophile. Consequently, Cp
1 will participate in P-DA reactions as a strong nucleophile without any nucleophilic activation. Note that the first DA reported in 1928 by Diels and Alder was the reaction between Cp
1 and maleic anhydride
2, a strong electrophile, ω = 3.24 eV [
1]. The inclusion of an electron-releasing (ER) group at the 1-position of Cp
7 markedly increases the nucleophilicity
N index of the corresponding Cp derivative, between 3.62 eV (
12, R = Me) and 4.65 (
14, R = NMe
2) eV. Consequently, it is expected that the DA reactions between these Cp derivatives and the acrolein:LA complexes will have a very strong polar character.
When a nucleophile approaches an electrophile along a polar process, some electron density is transferred from the nucleophilic to the electrophilic species. As a consequence, while the nucleophile loses some electron density, the electrophile gains it. These changes demand a reorganization of the electron density in both frameworks. In this context, the nucleophilic
and electrophilic
Parr functions [
32] have shown to be the most accurate and insightful tools for the study of the electron density redistribution along a polar process, being widely used for the analysis of the local reactivity in polar processes. The electrophilic
Parr functions of acrolein
7 and complexes
7-BH3 and
7-AlCl3, and the nucleophilic
Parr functions of Cp
1 and
13 are given in
Figure 3.
Analysis of the electrophilic
Parr functions of acrolein
7 indicates that the C5 carbon is the most electrophilic center of this molecule,
= 0.52. In fact, this position is twice activated than the carbonyl C7 carbon,
= 0.27 (for atom numbering see
Scheme 4). Coordination of the LA to the carbonyl O8 oxygen increases the electrophilic
Parr functions at the carbonyl C7 carbon, but the C5 carbon remains as the most electrophilic center of these LA complexes. Note that these changes do not modify the regioselectivity as in these LA complexes as the C6 carbon in electrophilic deactivated.
Analysis of the nucleophilic Parr functions of Cp 1 indicates that the C1 and C4 are symmetrically activated. These carbons are the most nucleophilic center of Cp 1, = 0.47. Interestingly, the inclusion of an ER–OMe at the C1 carbon does not alter the nucleophilic Parr function at the C4 carbon, = 0.47, but relocates the nucleophilic Parr function at the C1 and C2 carbons. Consequently, the C4 carbon becomes the most electrophilic center of 13.
Consequently, along a P-DA reaction involving non-symmetric reagents, the most favorable regioisomeric channels will be those associated with the two-center interaction between the most nucleophilic center of the diene framework, the C4 carbon, and the most electrophilic center of the electrophilic ethylene, the C5 carbon, in complete agreement with the experimental outcomes, and the energetic analysis of the different competitive reaction paths (see
Section 2.3) [
33].
2.2. Analysis of the Effects of the LAs and the Substitution on Cp 1 in the Electronic Structure of the Reagents
In order to get information about how the LA catalysts and the substituents present in Cp
1 can modify the electronic structure of the diene and ethylene frameworks, an electron localization function [
34] (ELF) and natural population analysis [
35,
36] (NPA) analysis of
7 and the LA complexes
7-BH3 and
7-AlCl3, and of Cps
1 and
13 was performed. The populations of the most relevant ELF valence basins of these reagents are gathered in
Table 2, while the ELF localization domains are represented in
Figure 4. The ELF-based Lewis-like structures and natural atomic charges are shown in
Chart 2.
ELF of acrolein
7 shows the presence of two disynaptic basins, V(C5,C6) and V’(C5,C6), integrating a total of 3.48 e, associated with the C5−C6 double bond, one V(C6,C7) disynaptic basin, integrating 2.22 e, associated with a C6−C7 single bond, one V(C7,O8) disynaptic basin, integrating 2.36 e, associated with a C7−O8 single bond, and two monosynaptic basins, V(O8) and V’(O8), integrating a total of 5.19 e, associated with the two lone pairs of the O8 oxygen. Two interesting conclusions can be obtained from this ELF analysis: i) the ethylene C5−C6 framework presents an identical electronic topology than that of ethylene
5 [11], indicating that at the GS the carbonyl group does not polarize the ethylene framework; and ii) the very low population of the carbonyl C7−O8 bonding regions, which corresponds with a C−O single bond, agrees with the high population of the two O8 lone pairs, indicating the high polarisation of the carbonyl group towards the O8 oxygen.
Coordination of the B or Al to the carbonyl O8 oxygen of acrolein
7 does not produce any remarkable change in the electronic structure of the acrolein framework of the two LA complexes (see
Figure 4). Only the C5–C6 bonding region is polarized towards the C6–C7, the C7–O8 bonding region being practically unaltered. Note that the changes in population of the disynaptic basins are lesser than 0.1 e. The most noticeable change is the redistribution of the electron density between the two O8 lone pairs. The absence of a V(O8,Metal) disynaptic basin at these LA complexes indicates that the metal centers of these LAs are not covalently bonded to the carbonyl O8 oxygen [
37].
ELF topology of Cp 1 shows the presence of two pairs of disynaptic basins, V(C1,C2) and V′(C1,C2), and V(C3,C4) and V′(C3,C4), integrating around 3.47 e each pair, associated with the C1−C2 and C3−C4 double bonds of Cp 1, and one V(C2,C3) disynaptic basin, integrating 2.17 e, associated with the C2−C3 single bond. It is interesting to note that the C1−C2 bonding region of Cp 1 is identical than that of the C5−C6 bonding region of acrolein 7, indicating that the carbonyl group does not polarize the C5−C6 double bond of acrolein 7. With the inclusion of the strong ER –OMe group on the C1 carbon of Cp 7 non-relevant changes in the electronic structure of 13 are observed. Only the population of the C1−C2 and C3−C4 bonding regions are increased below 0.1 e.
From these ELF topological analysis at the GS of the reagents we can conclude that neither the nature of the LA, nor the substitution of the Cp core substantially modify the electronic structure of the diene and ethylene frameworks.
Finally, an NPA of the natural charges at these selected compounds was made. The ELF-based Lewis-like structures together with natural atomic charges are given in
Chart 2. As expected, at acrolein
7, the carbonyl C7 carbon presents a high positive charge, +0.37 e, and the O8 oxygen presents a high negative charge, −0.52 e, as a consequence of the strong polarisation of the carbonyl C7−O8 bonding region. On the other hand, the C5 carbon presents a high negative charge, −0.35 e, as a consequence of the more electronegative character of the carbon nuclei than the two attached hydrogens. Coordination of the LA to the carbonyl O8 oxygen polarizes the C7−O8 framework, increasing the positive charge at the carbonyl C7 carbon. As a consequence, the ethylenic C5−C6 bonding region is some polarized towards the carbonyl group, decreasing slightly the negative charge at the C5 carbon. In spite of this, the C5 carbon remains negatively charged.
On the other hand, the NPA of the natural charge at Cp 7 indicates that the C2 and C3 carbons are more negatively charged than the C1 and C4 ones. The inclusion of the ER –OMe group at the C1 carbon breaks the symmetric electron density distribution found at Cp 1. Now the C1 and C2 carbon are more negatively charge than the C3 and C4 ones.
From the analysis of the global and local CDFT reactivity indices made in
Section 2.1 and the present analysis of the electronic structure at the GS of the reagents some appealing conclusions can be obtained: i) ELF analysis of the electronic structure of the reagents indicates that neither substitution on the diene or ethylene, nor the use of the LAs, modify the electronic structure of diene or ethylene framework participating in the DA reactions. In fact, the topology of the C5=C6−C7 framework of acrolein
7 is similar to that of the C1=C2−C3 framework of Cp
1; ii) NPA analysis of acrolein
7 indicates that only the carbonyl C7 carbon is positively charged, while the ethylene C5 carbon is negatively charged. Coordination of the LA to the carbonyl O8 oxygen does not modify substantially these behaviours; iii) NPA analysis of Cp
1 indicates that the C2 and C3 carbon are more negatively charged than the C1 and C4 ones; iv) inclusion of an ER –OMe group at the C1 carbon of Cp breaks the symmetric electron density distribution of Cp
1, making the C1 carbon of Cp
13 the most negatively charged carbon; v) consequently, analysis of the electronic structure at the GS of the reagents does not permit to explain the reactivity of these species participating in P-DA reaction; vi) analysis of the CDFT reactivity indices accounts for the participation of acrolein
7 and Cp
1 as electrophile and nucleophile in P-DA reactions, as well as the effects of the LA coordination to the electrophilic acrolein
7, and the ER substitution on Cp
1; vii) analysis of the electrophilic and nucleophilic Parr functions at the non-symmetric reagents accounts for the regioselectivity in these P-DA reactions. While the non-substituted C5 carbon of acrolein
7 is the most electrophilic center of this species, the non-substituted C4 carbon of Cp
1 is the most nucleophilic center of this diene; and viii) the present analysis supports the earlier observation that the electrophilicity and nucleophilicity cannot be related only with charges at the GS of the molecules, but with the propensity of changes on electron density resulting of the GEDT along a polar reaction [
38].
2.3. Study of the P-DA Reactions Between Cp 1 and the Series of LA Complexes
In order to shed light on the role of the LA catalysts on the DA reactions between Cp
1 and acrolein
7, the P-DA reactions of Cp
1 and five selected LA complexes of increased electrophilic character were first studied (see
Table 1). For a comparative analysis, the P-DA reactions between Cp
1 and acrolein
7 was also studied; the corresponding results are given in the
Section S2 of the Supplementary Material. Due to the non-symmetry of these LA complexes, two stereoisomeric reaction paths, the
endo and the
exo, are feasible (see
Section S1 of the Supplementary Material). Analysis of the stationary points involved in these P-DA reactions indicates that they take place along a one-step mechanism. Consequently, the reagents, one molecular complex (MC),
MC-LA, two stereoisomeric TSs,
TSn-LA and
TSx-LA, and the corresponding cycloadducts (CA),
CAn-LA and
CAx-LA, were localized and characterized (
Scheme 5). Relative gas phase electronic energies are given in
Table 3. Total energies are given in
Table S2 in the Supplementary Material.
An exploration of the reaction paths between the separated reagents and the TSs allowed finding a series of MCs in which the two reagents are close in a parallel rearrangement. From these MCs, those associated to the endo reaction paths were selected as the energy reference. These MC-LAs are between 1.9 (MC-AlMe3) and 4.2 (MC-AlCl3) kcal·mol−1 more stable than separated reagents, being minimum in their corresponding potential energy surfaces (PESs). The activation energies associated to the two stereoisomeric reaction paths range from 13.0 to 5.2 kcal·mol−1 for the endo TSs, and from 13.0 to 5.6 kcal·mol−1 for the exo ones. The five P-DA reactions are exothermic between 13.8 and 14.8 kcal·mol−1.
Some appealing conclusions can be obtained from these energy results: i) the activation energies associated to these P-DA reactions are between 6.0 and 13.8 kcal·mol
−1 lower than that associated with the P-DA reaction between Cp
1 and acrolein
7 (see
Scheme S4 in the Supplementary Material); ii) a good correlation between the electrophilicity ω index of the LA complex at the corresponding activation energy can be established. The more electrophilic the LA complex is, the more rapid the P-DA; iii) these P-DA reactions are low
endo stereoselective; the
endo TSs are found between 0.2 and 0.4 kcal·mol
−1 lesser energetic than the
exo ones; and iv) these P-DA reactions are exothermic between 13.8 and 14.7 kcal·mol
−1. Consequently, coordination of the LA to the carbonyl oxygen of acrolein
7 only modifies the kinetics of the reactions.
The geometries of two stereoisomeric TSs involved in the P-DA reactions of Cp
1 with the series of LA complexes are given in
Figure 3. From the distance between the C4−C5 and C1−C6 interacting carbons at the two stereoisomeric TSs some appealing conclusions can be obtained: i) in all TSs, the C4−C5 distances involving the most C5 electrophilic carbon of the LA complexes is shorter than the C1−C6 ones; ii) the C4−C5 distances vary is the narrow range of 1.97–2.00 Å, while the C1−C6 distances vary between 2.64–2.88 Å. Consequently, all TSs correspond to high asynchronous single bond formation processes; iii) an increase of the C1−C6 distance is observed with the increase of the electrophilic character of the LA complex; iv) the
endo TSs are slightly more advance and more asynchronous than the
exo TSs; v) the C1−C6 distances is slightly shorter at the
endo TSs than at the
exo ones. This behaviour is a consequence of the most favorable electrostatic interactions appearing in the zwitterionic
endo TSs than at the
exo ones; and vi) in spite of the high asynchronous character of
TSn-AlCl3, intrinsic reaction coordinate (IRC) analysis of this TS indicates that it is associates to a one-step mechanism.
The polar nature of these LA catalyzed DA reactions was evaluated by computing the GEDT at the corresponding TSs. Reactions with GEDT values of 0.0 e correspond to non-polar processes, while values higher than 0.2 e correspond to polar processes. The computed GEDT values at the corresponding TSs varies from 0.27 e at
TSn-BH3 to 0.39 e at
TSn-AlCl3, indicating the high polar character of these DA reactions (see
Figure 5). Note that the GEDT at the
endo TSn associated to the uncatalyzed DA reaction is 0.15 e (see
Section S2 in the Supplementary Material). A slightly higher GEDT is found at the
endo TSs that at the
exo ones, as consequent of a more favorable electrostatic interaction at the zwitterionic
endo TSs.
As expected, a very good linear correlation between the activation energies and the GEDT computed at the six TSs is found, R
2 = 0.94 (see
Figure 6). The values associated to the 32CA reaction of Cp
1 with ethylene
5 and acrolein
7 are also included. When the non-polar DA reaction with ethylene
7 and the most P-DA reaction with
7-AlCl3 are excluded, the R
2 reaches the value of 0.99. The slope of the linear correlation, −46.8 (not shown in
Figure 6), can be associated with the extent of activation energy decrease in these P-DA reactions with the increase of the GEDT taking place at the TS.
2.4. Study of the P-DA Reactions Between Substituted Cps 12–14 and Complex 7-BF3
After to study the effect of the increase electrophilicity of acrolein
7 with the coordination to a LA in the before section, the role of the nucleophilic activation of the diene on the activation energy and selectivities was explored. To this end, the P-DA reactions of three 1-subtituted Cps,
12 (R = Me),
13 (R = OMe), and
14 (R = NMe
2), of increased nucleophilic character (see
Table 1), with complex
7-BF3 were studied. Because of the non-symmetry of these 1-subtituted Cps, together with the
endo/
exo stereoisomeric reaction paths, two regioisomeric reaction paths are feasible (see
Section S1 of Supplementary Material). Consequently, the four competitive reaction paths were explored (see
Scheme 6 and
Scheme 7). Interestingly, due to the presence of the strong ER –OMe and –NMe
2 groups in Cps
13 and
14, respectively, allowing the stabilization of the corresponding zwitterionic intermediates, a two-step mechanism for the most favorable
ortho regioisomeric reaction paths were found. Consequently, two TSs and one zwitterionic intermediate were located and characterized for the
ortho regioisomeric reaction paths of
13 and
14. Relative gas phase electronic energies are given in
Table 4. Total energies are given in
Table S3 in the Supplementary Material.
Some appealing conclusions can be obtained from the relative energy given in
Table 4: i) the most favorable reactive channels corresponds to
endo channels via
TSon-C-BF3 or
TS1on-X-BF3; ii) a strong reduction of the activation energy is found with the increase of the nucleophilic character of the Cp derivative. In fact,
TS1on-O-BF3 and
TS1on-N-BF3 are found below reagents. This behaviour demands the characterisation of the corresponding MCs,
MC-O-BF3 and
MC-N-BF3, in order to obtain positive activation energies; ii) while the P-DA reactions of Cps
12 and
14 presents low
endo selectivity as
TS1ox-X-BF3 are 0.9 and 0.7 kcal·mol
−1 higher in energy than
TS1on-X-BF3, respectively, the P-DA reactions of the methoxy derivative
13 presents a high
endo selectivity as
TS1ox-O-BF3 is 2.7 kcal·mol
−1 higher in energy than
TS1on-O-BF3; iii) these P-DA reactions presents complete regioselectivity as
TSmn-X-BF3 are 5.7 (
12), 14.1 (
13) and 23.4 (
14) kcal·mol
−1 higher in energy than
TS1on-X-BF3. The regioselectivity of these P-DA reactions increases notably with the increase of the polar character of the reaction (see later). Interestingly, while the activation energies associated to
TS1on-O-BF3 and
TS1ox-N-BF3 decrease with the increase of the nucleophilic character of the Cp derivative, the activation energies associated to
TSmn-O-BF3 and
TSmx-N-BF3 increase; iv) formation of the zwitterionic intermediates is exothermic by 6.6 (
ZWon-O-BF3) and 20.9 (
ZWon-N-BF3) kcal·mol
−1; v) in spite of the high stabilization of the zwitterionic intermediates
ZWon-O-BF3 and
ZWox-O-BF3, the low activation energies associated with the ring closure of these species, lesser that 1.3 kcal·mol
−1, makes they unobservable. Consequently, in gas phase there are not any difference between the
two-stage one-step mechanism [
39] and the two-step one associated with the P-DA reactions of Cp
1 and Cp
13 with complex
7-BF3 (see
Section 2.7 and
Section 2.8).
The geometries of stereoisomeric TSs and intermediates involved in the P-DA reactions of Cp
13 with complex
7-BF3 are given in
Figure 7, while those associated with the P-DA reactions of Cps
12 and
14 are given in
Figures S2 and S3 of the Supplementary Material. The lengths of the C−C forming bonds are given in
Table 5. Only the parameter associated with the P-DA reaction of Cp
13 will be herein commented on. From the distances between the C4−C5 and C1−C6 interacting carbons at the TSs and intermediates shown in
Figure 5 some appealing conclusions can be obtained: i) the most favorable
TS1on-O-BF3 and
TS1ox-O-BF3 are slightly more delayed and more asynchronous than
TSn-BF3 and
TSx-BF3; ii) at
ZWon-O-BF3 and
ZWox-O-BF3 the C4−C5 distances, 1.632 and 1.619 Å, respectively, indicate that the C4−C5 single bond has been already formed [
13], while the C1−C6 distances remain larger than 2.56 Å. Note that while the length of C−C single bond at numerous organic compounds is 1.54 Å, that at reaction intermediates is slightly longer, 1.6 Å; iii) at the TSs associated with the ring closure, the C1−C6 distances are 2.185 Å at
TS2on-O-BF3 and 2.236 Å at
TS2ox-O-BF3. These distances indicate that the formation of the news C−C single bond does not begun yet [
13]; iv) the C1−C5 and C4−C6 distances at the regioisomeric TSs, 1.935 and 2.514 Å at
TSmn-O-BF3 and 1.934 and 2.582 Å
TSmx-O-BF3, respectively, indicate that these TSs are more advanced and less asynchronous than the regioisomeric
TS1on-O-BF3 and
TS1ox-O-BF3.
The GEDT values at the TSs associated with the nucleophilic attacks of the Cps
12–14 on the complex
7-BF3 ranges for 0.35 e (
TSmn-C-BF3) to 0.41e (
TS1on-O-BF3), while at the zwitterionic intermediates the corresponding values are found between 0.55 e (
ZWox-O-BF3) to 0.79 e (
ZWon-N-BF3). Some appealing conclusions can be obtained from the GEDT values given in
Table 5: i) The high GEDT values found in these LA catalyzed reactions, higher than 0.35 e, account for the high polar character and low activation energies, lesser than 5 kcal·mol
−1, associated with these P-DA reactions; ii) The GEDT found at the most favorable
TS1on-N-BF3, 0.40 e, is slightly lower than that at
TS1on-O-BF3, 0.41 e. This finding is a consequence of the fact that the GEDT values depend on the nucleophilic and electrophilic behaviours of the two reagents, but also of the distance between the two interacting frameworks. Note that the C4−C5 distances at the two TSs are 2.111 at Å
TS1on-O-BF3 and 2.328 at Å
TS1on-N-BF3; i.e., the more advanced TS, the higher the GEDT is. Note that the maximum of GEDT along the reaction path is found at the ZW intermediates: 0.60 e at
ZWon-O-BF3 and 0.79 e at
ZWon-N-BF3. At these intermediates, which present a similar C4−C5 distances, ca 1.60 Å, the GEDT values account for the more nucleophilic character of Cp
14 than Cp
13 (see
Table 1); and iii) The GEDT values at the most favorable
endo TSs and intermediates are slightly higher than that at the
exo ones. The favorable electrostatic interactions appearing in the
endo approach mode favors the GEDT and diminishes the energy of the
endo TS. Interestingly, at the more polar
endo structure, the C1−C6 distance is larger; iv) at the very unfavorable regioisomeric
meta TSs, the GEDT is only slightly lower than that at the
ortho TSs, but energetically are more unfavorable, even to the
endo TS associated with P-DA between Cp
1 and complex
7-BF3 (see
Table 5). This finding that was already highlighted in 2010, supports the concept of GEDT given in 2014 [
13], since the electron density fluxes from the nucleophile to the electrophile, being not much dependent of the orientation of the approach of the reagents. As earlier was proposed, this finding goes against the arrows model for the “electron transfer” given in all organic textbooks.
An appealing question arises from the present GEDT analysis: why the activation energy decreases notably with the increase of GEDT at the more favorable ortho regioisomeric reaction paths, but it increases with the increase of GEDT at the more unfavorable meta regioisomeric reaction paths?
To answer this question, we need to consider two pertinent findings. First, based on the ELF topological analysis of the bonding changes along the reaction path of numerous organic reactions, in 2014 Domingo proposed a model for the C−C bond formation in organic reactions, in which they are formed by the C-to-C coupling of two
pseudoradical centers created along the reaction paths [
13]. This model was applicable even in ionic Diels–Alder reactions. In 2013, Domingo had already proposed the Parr functions [
33], as the changes in spin electron density after the transfer of one electron from the nucleophile to the electrophile, to explain the local reactivity in polar reactions. Second, the high activation energy found in non-polar reactions was associated with the energy demanded for the depopulation of the C−C double bonds, which is required for the formation of the two aforementioned
pseudoradical centers [
11]. Thus, the acceleration found in polar reactions was associated to the favorable electronic effects caused by the electron density transfer in the bonding changes [
38].
Figure 8 shows the ELF localization domains of the regioisomeric
TS1on-O-BF3 and
TSmn-O-BF3 involved in the P-DA reaction between Cp
12 and complex
7-BF3. ELF of
TS1on-O-BF3 shows the presence of two monosynaptic basins, V(C4) and V(C5), integrating 0.43 and 0.15 e, respectively, associated to the C4 and C5
pseudoradical centers demanded for the subsequent C4−C5 single bond formation. On the other hand, ELF of
TSmn-O-BF3 also shows the presence of two monosynaptic basins, V(C1) and V(C5), integrating 0.63 and 0.44 e, respectively, associated to the C1 and C5
pseudoradical centers demanded for the subsequent C1−C5 single bond formation.
Thus, while the C4 and C5
pseudoradical centers present at the more favorable
TS1on-O-BF3 correspond with the most nucleophilic and the most electrophilic centers of Cp
13 and complex
7-BF3, respectively, being their formation favored by the GEDT (see Parr functions in
Figure 1), the formation of the C1
pseudoradical center at
TSmn-O-BF3 is not favored by the GEDT as it mainly directs the electron density towards the C4 carbon.
Consequently, along the most favorable regioisomeric reaction path, the GEDT facilitates the bonding changes demanded to reach
TS1on-O-BF3, such as the Parr functions predict, but at the regioisomeric
TSmn-O-BF3, where the two reacting molecules are approximated in a contrary orientation, the formation of the C1
pseudoradical center is not facilitated by the GEDT, as it mainly directs the electron density to the C4 carbon. Therefore,
TSmn-O-BF3 is energetically more unfavorable [
33]. When more polar the DA reaction, higher the difference energy between two the regioisomeric paths is. This behaviour allows explaining the increase of the regioselectivity in LA catalyzed DA reactions.
2.8. Stablishing the Similarity Between Two-Stage One-Step and Two-Step Mechanisms in LA Catalyzed DA Reactions
In 2008 Domingo proposed the concept of
two-stage one-step mechanisms to describe those reactions in which formation of the two C−C single bonds takes place in a non-concerted process but in a single kinetic step [
39]. At the high asynchronous TSs associated to these one-step reactions only one C−C single bond is being formed along a two-center interaction, while the formation of the second C−C single bond begins at the end of the reaction path, when the formation of the first C−C single bond is practically formed [
39].
In order to stablish the similarity between the
two-stage one-step and the two-step mechanisms, a comparative analysis of the ELF of the TSs and intermediate involved in the
endo reaction path of the two-step P-DA reaction between Cp
13 and complex
7-BF3, and some selected structures of the IRC of
endo reaction path of the
two-stage one-step P-DA reaction between Cp
1 and complex
7-BF3, was performed.
Figure 11 shows the position of the ELF attractors of
TS1on-O-BF3, ZWon-O-BF3 and
TS2on-O-BF3. ELF analysis of the
endo reaction path of the
two-stage one-step P-DA reaction between Cp
1 and complex
7-BF3 is given in
Section S3 of the Supplementary Material.
ELF of
TS1on-O-BF3 shows the presence of two monosynaptic basins, V(C4) and V(C5), integrating 0.43 and 0.17 e, respectively (see
Figure 11). As expected, these monosynaptic basins appear at the most nucleophilic center of Cp
13, the C4 carbon, and at the most electrophilic center of complex
7-BF3, the C5 carbon. ELF of
TS1on-O-BF3 is closely to that of
TSn-BF3 (see
Table S1 in Supplementary Material). The population of V(C4) and V(C5) monosynaptic basins is slightly higher in
TSn-BF3, 0.48 and 0.33 e, respectively, as this TS is more advanced than
TS1on-O-BF3; the C4−C5 distance at these TSs is 2.111 Å at
TS1on-O-BF3 and 1.977 Å at
TSn-BF3. Consequently,
TS1on-O-BF3 and
TSn-BF3 are associated to the same chemical process; i.e., the nucleophilic attack of Cp
1 or Cp
13 to complex
7-BF3. Sometimes, these high asynchronous TSs have been associated to a Michael addition [
42].
ELF of ZWon-O-BF3 shows the disappearance of V(C4) and V(C5) monosynaptic basins, and the appearance of a new V(C4,C5) disynaptic basin integrating 1.59 e, indicating the formation of the first C4−C5 single bond. ELF of ZWon-O-BF3 is closely to that of the last structure of Phase VII, in which the V(C4,C5) disynaptic basin integrates 1.58 e. The length of the C4−C5 single bond at these species is 1.632 Å at ZWon-O-BF3 and 1.624 Å at the last structure of Phase VII.
Finally, ELF of
TS2on-O-BF3 shows the presence of two monosynaptic basins, V(C1) and V(C6), integrating 0.26 and 0.44 e, respectively. At this TS, the population of the new V(C4,C5) disynaptic basin has been increased by only 0.09 e. ELF of
TS2on-O-BF3 is also closely to that of the structure of
S9 (see
Figure 11), in which the population of V(C1) and V(C6) monosynaptic basins is slightly lower, 0.18 and 0.33 e, respectively; the C1−C6 distance at these species is 2.185 Å at
TS1on-O-BF3 and 2.229 Å at
S9.
Consequently, this ELF comparative analysis allows establishing that the electronic structures and geometries of TSn-BF3, and the two selected points of the IRC defining the two-stage one-step mechanism are very closely to those of TS1on-O-BF3, ZWon-O-BF3 and TS2on-O-BF3, asserting a similar bonding changes along the two reaction mechanisms.
Thus, where is the difference between both mechanisms? Both reactions are polar processes associated to the nucleophilic attack of the C4 carbon of Cp frameworks to the C5 carbon of complex 7-BF3. The GEDT increases along this polar process, reaching the maximum value at ZWon-O-BF3, 0.60 e, and S9, 0.32 e. The high GEDT found at the zwitterionic intermediate is a consequence of the presence of the strong ER -OMe group at the Cp framework that not only favors the GEDT, but also stabilize thermodynamically the corresponding intermediate, thus becoming as a stationary point along the reaction path, a behavior that is not so effective at the structure S9. Note that relative energies of ZWon-O-BF3 and S9, with respect to the separated reagents are −6.6 and 6.6 kcal·mol−1.
So, the thermodynamic stabilization of some structure after the formation of the first C−C single bond along the reaction path is able to change the molecular mechanism from a two-stage one-step mechanism to two-step one. In that study, the change of the weak ER methyl group in Cp 12 by a strong ER -OMe group in Cp 13 changes the molecular mechanism from one-step to a two-step one. Sometimes, the use of polar solvent in the geometry optimizations, the use of DFT functional such as the M0-6X, which favors the polar process, of the use of larger basis sets, able to stabilize a zwitterionic intermediate, can also changes the molecular mechanism from a two-stage one-step to a two-step one, but this behaviour has no significance as the chemical characteristics of these reactions, i.e., reactions rates and selectivities are generally established at the TSs associated with the initial nucleophilic/electrophilic interaction.