Phosphorus Dendrimers as Nanotools against Cancers †
Abstract
:1. Introduction
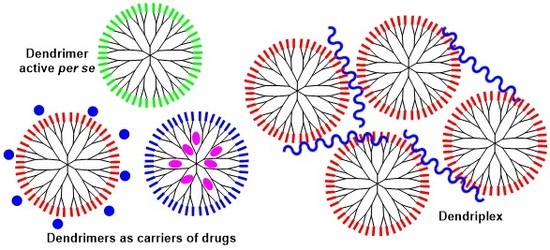
2. Phosphorus Dendrimers as Anticancer Drugs by Themselves
2.1. Phosphorhydrazone Dendrimers Functionalized by Organic Derivatives
2.2. Phosphorhydrazone Dendrimers Functionalized by Metal Complexes
3. Phosphorus Dendrimers as Carriers of Anticancer “Drugs”
3.1. Phosphorhydrazone Dendrimers as Carriers of Known Anticancer Drugs
3.2. Phosphorhydrazone Dendrimers as Carriers of Therapeutic Oligonucleotides
3.3. Associations of Therapeutic Agents against Cancers
4. Indirect Anticancer Activity of Phosphorus Dendrimers
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. I. Gelation. J. Am. Chem. Soc. 1941, 63, 3083–3090. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. II. Trifunctional Branching Units. J. Am. Chem. Soc. 1941, 63, 3091–3096. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, F.; Vögtle, F. “Cascade-” and “Nonskid-chain-like” syntheses of molecular cavity topologies. Synthesis 1978, 78, 155–158. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers—Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Caminade, A.-M.; Turrin, C.-O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. Dendrimers: Towards Catalytic, Material and Biomedical Uses; John Wiley & Sons Ltd.: Chichester, UK, 2011; p. 538. ISBN 978-0-470-74881-7. [Google Scholar]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst dendrimers—Molecular level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem. Int. Edit. Engl. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- de Brabander van den Berg, E.M.M.; Meijer, E.W. Poly(Propylene Imine) Dendrimers—Large-Scale Synthesis by Hetereogeneously Catalyzed Hydrogenations. Angew. Chem. Int. Edit. Engl. 1993, 32, 1308–1311. [Google Scholar] [CrossRef] [Green Version]
- Caminade, A.M. Inorganic dendrimers: Recent advances for catalysis, nanomaterials, and nanomedicine. Chem. Soc. Rev. 2016, 45, 5174–5186. [Google Scholar] [CrossRef]
- Rebrov, E.A.; Muzafarov, A.M.; Papkov, V.S.; Zhdanov, A.A. Three-dimensionally propagating polyorganosiloxanes. Doklady Akademii Nauk SSSR 1989, 309, 376–380. [Google Scholar]
- Rengan, K.; Engel, R. Phosphonium cascade molecules. J. Chem. Soc. Chem. Commun. 1990, 1084–1085. [Google Scholar] [CrossRef]
- Majoral, J.P.; Caminade, A.M. Dendrimers containing heteroatoms (Si, P, B, Ge, or Bi). Chem. Rev. 1999, 99, 845–880. [Google Scholar] [CrossRef]
- Zhou, L.L.; Roovers, J. Synthesis of novel carbosilane dendritic macromolecules. Macromolecules 1993, 26, 963–968. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.M.; Lahana, R.; Majoral, J.P. A general synthetic strategy for neutral phosphorus-containing dendrimers. Angew. Chem. Int. Edit. Engl. 1994, 33, 1589–1592. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.M.; Majoral, J.P. Synthesis and reactivity of unusual phosphorus dendrimers—A useful divergent growth approach up to the 7th generation. J. Am. Chem. Soc. 1995, 117, 3282–3283. [Google Scholar] [CrossRef]
- Slany, M.; Bardaji, M.; Casanove, M.J.; Caminade, A.M.; Majoral, J.P.; Chaudret, B. Dendrimer surface-chemistry—Facile route to polyphosphines and their gold complexes. J. Am. Chem. Soc. 1995, 117, 9764–9765. [Google Scholar] [CrossRef]
- Lartigue, M.L.; Donnadieu, B.; Galliot, C.; Caminade, A.M.; Majoral, J.P.; Fayet, J.P. Large dipole moments of phosphorus-containing dendrimers. Macromolecules 1997, 30, 7335–7337. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.M.; Majoral, J.P. Synthesis of bowl-shaped dendrimers from generation 1 to generation 8. J. Organomet. Chem. 1997, 529, 51–58. [Google Scholar] [CrossRef]
- Galliot, C.; Larre, C.; Caminade, A.M.; Majoral, J.P. Regioselective stepwise growth of dendrimer units in the internal voids of a main dendrimer. Science 1997, 277, 1981–1984. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Laurent, R. Homogeneous catalysis with phosphorus dendrimer complexes. Coord. Chem. Rev. 2019, 389, 59–72. [Google Scholar] [CrossRef]
- Caminade, A.M.; Majoral, J.P. Nanomaterials based on phosphorus dendrimers. Acc. Chem. Res. 2004, 37, 341–348. [Google Scholar] [CrossRef]
- Caminade, A.-M. Phosphorus dendrimers for nanomedicine. Chem. Commun. 2017, 53, 9830–9838. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Turrin, C.-O.; Majoral, J.-P. Phosphorus Dendrimers in Biology and Nanomedicine. Synthesis, Characterization, and Properties; Pan Stanford Publishing: Singapore, 2019; p. 372. ISBN 9789814774338. [Google Scholar]
- Solassol, J.; Crozet, C.; Perrier, V.; Leclaire, J.; Beranger, F.; Caminade, A.M.; Meunier, B.; Dormont, D.; Majoral, J.P.; Lehmann, S. Cationic phosphorus-contain ing dendrimers reduce prion replication both in cell culture and in mice infected with scrapie. J. Gen. Virol. 2004, 85, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Wasiak, T.; Ionov, M.; Nieznanski, K.; Nieznanska, H.; Klementieva, O.; Granell, M.; Cladera, J.; Majoral, J.P.; Caminade, A.M.; Klajnert, B. Phosphorus Dendrimers Affect Alzheimer’s (A beta(1-28)) Peptide and MAP-Tau Protein Aggregation. Mol. Pharm. 2012, 9, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Perez-Anes, A.; Rodrigues, F.; Caminade, A.M.; Stefaniu, C.; Tiersch, B.; Turrin, C.O.; Blanzat, M. Influence of Structural Parameters on the Self-Association Properties of Anti-HIV Catanionic Dendrimers. ChemPhysChem 2015, 16, 3433–3437. [Google Scholar] [CrossRef] [PubMed]
- Vacas-Cordoba, E.; Bastida, H.; Pion, M.; Hameau, A.; Ionov, M.; Bryszewska, M.; Caminade, A.M.; Majoral, J.P.; Munoz-Fernandez, M.A. HIV-Antigens Charged on Phosphorus Dendrimers as Tools for Tolerogenic Dendritic Cells-Based Immunotherapy. Curr. Med. Chem. 2014, 21, 1898–1909. [Google Scholar] [CrossRef]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournie, J.J.; Cantagrel, A.; et al. A Phosphorus-Based Dendrimer Targets Inflammation and Osteoclastogenesis in Experimental Arthritis. Science Transl. Med. 2011, 3, 11. [Google Scholar] [CrossRef]
- Fruchon, S.; Caminade, A.M.; Abadie, C.; Davignon, J.L.; Combette, J.M.; Turrin, C.O.; Poupot, R. An Azabisphosphonate-Capped Poly(phosphorhydrazone) Dendrimer for the Treatment of Endotoxin-Induced Uveitis. Molecules 2013, 18, 9305–9316. [Google Scholar] [CrossRef] [Green Version]
- Blattes, E.; Vercellone, A.; Eutamene, H.; Turrin, C.O.; Theodorou, V.; Majoral, J.P.; Caminade, A.M.; Prandi, J.; Nigou, J.; Puzo, G. Mannodendrimers prevent acute lung inflammation by inhibiting neutrophil recruitment. Proc. Natl. Acad. Sci. USA 2013, 110, 8795–8800. [Google Scholar] [CrossRef] [Green Version]
- Katir, N.; Majoral, J.P.; El Kadib, A.; Caminade, A.M.; Bousmina, M. Molecular and Macromolecular Engineering with Viologens as Building Blocks: Rational Design of Phosphorus-Viologen Dendritic Structures. Eur. J. Org. Chem. 2012, 269–273. [Google Scholar] [CrossRef]
- Lazniewska, J.; Janaszewska, A.; Milowska, K.; Caminade, A.M.; Mignani, S.; Katir, N.; El Kadib, A.; Bryszewska, M.; Majoral, J.P.; Gabryelak, T.; et al. Promising Low-Toxicity of Viologen-Phosphorus Dendrimers against Embryonic Mouse Hippocampal Cells. Molecules 2013, 18, 12222–12240. [Google Scholar] [CrossRef]
- Ciepluch, K.; Katir, N.; El Kadib, A.; Felczak, A.; Zawadzka, K.; Weber, M.; Klajnert, B.; Lisowska, K.; Caminade, A.M.; Bousmina, M.; et al. Biological Properties of New Viologen-Phosphorus Dendrimers. Mol. Pharm. 2012, 9, 448–457. [Google Scholar] [CrossRef]
- Zhao, G.; Yu, T.; Wang, R.; Wang, X.; Jing, Y. Synthesis and structure–activity relationship of ethacrynic acid analogues on glutathione-s-transferase P1-1 activity inhibition. Bioorg. Med. Chem. 2005, 13, 4056–4062. [Google Scholar] [CrossRef] [PubMed]
- Hansson, J.; Berhane, K.; Castro, V.M.; Jungnelius, U.; Mannervik, B.; Ringborg, U. Sensitization of human-melanoma cells to the cytotoxic effect of Melphalan by the glutathione transferase inhibitor ethacrynic acid. Cancer Res. 1991, 51, 94–98. [Google Scholar] [PubMed]
- Oakley, A.J.; Rossjohn, J.; LoBello, M.; Caccuri, A.M.; Federici, G.; Parker, M.W. The three-dimensional structure of the human Pi class glutathione transferase P1-1 in complex with the inhibitor ethacrynic acid and its glutathione. Biochemistry 1997, 36, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Gast, S.M.; Endo Lu, T.; Carson, D.; Schmidt-Wolf, G.H. In vivo efficacy of the diuretic agent ethacrynic acid against multiple myeloma. Leukemia Res. 2012, 36, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; El Brahmi, N.; El Kazzouli, S.; Eloy, L.; Courilleau, D.; Caron, J.; Bousmina, M.M.; Caminade, A.M.; Cresteil, T.; Majoral, J.P. A novel class of ethacrynic acid derivatives as promising drug-like potent generation of anticancer agents with established mechanism of action. Eur. J. Med. Chem. 2016, 122, 656–673. [Google Scholar] [CrossRef]
- El Brahmi, N.; Mignani, S.M.; Caron, J.; El Kazzouli, S.; Bousmina, M.M.; Caminade, A.M.; Cresteil, T.; Majoral, J.P. Investigations on dendrimer space reveal solid and liquid tumor growth-inhibition by original phosphorus-based dendrimers and the corresponding monomers and dendrons with ethacrynic acid motifs. Nanoscale 2015, 7, 3915–3922. [Google Scholar] [CrossRef]
- El Brahmi, N.; El Kazzouli, S.; Mignani, S.; Laurent, R.; Ladeira, S.; Caminade, A.M.; Bousmina, M.; Majoral, J.P. Symmetrical and unsymmetrical incorporation of active biological monomers on the surface of phosphorus dendrimers. Tetrahedron 2017, 73, 1331–1341. [Google Scholar] [CrossRef]
- Rozencweig, M.; von Hoff, D.D.; Slavik, M.; Muggia, F.M. Cis-diamminedichloroplatinum (II). A new anticancer drug. Ann. Intern. Med. 1977, 86, 803–812. [Google Scholar] [CrossRef]
- Bellon, S.F.; Coleman, J.H.; Lippard, S.J. DNA unwinding produced by site-specific intrastrand cross-links of the antitumor drug cis-diamminedichloroplatinum(II). Biochemistry 1991, 30, 8026–8035. [Google Scholar] [CrossRef]
- van Zutphen, S.; Reedijk, J. Targeting platinum anti-tumour drugs: Overview of strategies employed to reduce systemic toxicity. Coord. Chem. Rev. 2005, 24, 2845–2853. [Google Scholar] [CrossRef]
- Gasser, G.; Oh, I.; Metzler-Nolte, N. Organometallic Anticancer compounds. J. Med. Chem. 2011, 54, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Maraval, V.; Laurent, R.; Caminade, A.M.; Majoral, J.P. Phosphorus-containing dendrimers and their transition metal complexes as efficient recoverable multicenter homogeneous catalysts in organic synthesis. Organometallics 2000, 19, 4025–4029. [Google Scholar] [CrossRef]
- Zablocka, M.; Hameau, A.; Caminade, A.M.; Majoral, J.P. “Cage-Like” Phosphines: Design and Catalytic Properties. Adv. Synth. Catal. 2010, 352, 2341–2358. [Google Scholar] [CrossRef]
- Servin, P.; Laurent, R.; Dib, H.; Gonsalvi, L.; Peruzzini, M.; Majoral, J.P.; Caminade, A.M. Number of terminal groups versus generation of the dendrimer, which criteria influence the catalytic properties? Tetrahedron Lett. 2012, 53, 3876–3879. [Google Scholar] [CrossRef]
- Servin, P.; Laurent, R.; Gonsalvi, L.; Tristany, M.; Peruzzini, M.; Majoral, J.P.; Caminade, A.M. Grafting of water-soluble phosphines to dendrimers and their use in catalysis: Positive dendritic effects in aqueous media. Dalton Trans. 2009, 4432–4434. [Google Scholar] [CrossRef] [PubMed]
- Servin, P.; Laurent, R.; Tristany, M.; Romerosa, A.; Peruzzini, M.; Garcia-Maroto, F.; Majoral, J.-P.; Caminade, A.-M. Dual properties of water-soluble Ru-PTA complexes of dendrimers: Catalysis and interaction with DNA. Inorg. Chim. Acta 2018, 470, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Ouali, A.; Laurent, R.; Caminade, A.M.; Majoral, J.P.; Taillefer, M. Enhanced catalytic properties of copper in O- and N-arylation and vinylation reactions, using phosphorus dendrimers as ligands. J. Am. Chem. Soc. 2006, 128, 15990–15991. [Google Scholar] [CrossRef]
- El Brahmi, N.; El Kazzouli, S.; Mignani, S.M.; Essassi, E.; Aubert, G.; Laurent, R.; Caminade, A.M.; Bousmina, M.M.; Cresteil, T.; Majoral, J.P. Original Multivalent Copper(II)-Conjugated Phosphorus Dendrimers and Corresponding Mononuclear Copper(II) Complexes with Antitumoral Activities. Mol. Pharm. 2013, 10, 1459–1464. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; El Brahmi, N.; Cangiotti, M.; Coppola, C.; Buccella, F.; Cresteil, T.; Mignani, S.; Caminade, A.M.; Costes, J.P.; Majoral, J.P. Comparative EPR studies of Cu(II)-conjugated phosphorous-dendrimers in the absence and presence of normal and cancer cells. Rsc Adv. 2014, 4, 36573–36583. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Zibarov, A.; Diaz, E.C.; Hameau, A.; Klausen, M.; Ching, K.M.-C.; Majoral, J.-P.; Verlhac, J.-B.; Mongin, O.; Blanchard-Desce, M. Fluorescent phosphorus dendrimers excited by two photons: Synthesis, two-photon absorption properties and biological uses. Beilstein J. Org. Chem. 2019, 15, 2287–2303. [Google Scholar] [CrossRef]
- Mignani, S.; El Brahmi, N.; Eloy, L.; Poupon, J.; Nicolas, V.; Steinmetz, A.; El Kazzouli, S.; Bousmina, M.M.; Blanchard-Desce, M.; Caminade, A.M.; et al. Anticancer copper(II) phosphorus dendrimers are potent proapoptotic Bax activators. Eur. J. Med. Chem. 2017, 132, 142–156. [Google Scholar] [CrossRef]
- Mignani, S.M.; El Brahmi, N.; El Kazzouli, S.; Laurent, R.; Ladeira, S.; Caminade, A.-M.; Pedziwiatr-Werbicka, E.; Szewczyk, E.M.; Bryszewska, M.; Bousmina, M.M.; et al. Original Multivalent Gold(III) and Dual Gold(III)-Copper(II) Conjugated Phosphorus Dendrimers as Potent Antitumoral and Antimicrobial Agents. Mol. Pharm. 2017, 14, 4087–4097. [Google Scholar] [CrossRef]
- Chen, L.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P. Metal-based phosphorus dendrimers as novel nanotherapeutic strategies to tackle cancers: A concise overview. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1577. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1. [Google Scholar] [CrossRef]
- Cardona, C.M.; McCarley, T.D.; Kaifer, A.E. Synthesis, electrochemistry, and interactions with beta-cydodextrin of dendrimers containing a single ferrocene subunit located “off-center”. J. Org. Chem. 2000, 65, 1857–1864. [Google Scholar] [CrossRef]
- Morikawa, A.; Kakimoto, M.A.; Imai, Y. Convergent Synthesis of Siloxane Starburst Dendrons and Dendrimers via Hydrosilylation. Macromolecules 1992, 25, 3247–3253. [Google Scholar] [CrossRef]
- Hameau, A.; Fuchs, S.; Laurent, R.; Majoral, J.P.; Caminade, A.M. Synthesis of dye/fluorescent functionalized dendrons based on cyclotriphosphazene. Beilstein J. Org. Chem. 2011, 7, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Majoral, J.P. Bifunctional Phosphorus Dendrimers and Their Properties. Molecules 2016, 21, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caminade, A.-M.; Ouali, A.; Hameau, A.; Laurent, R.; Rebout, C.; Delavaux-Nicot, B.; Turrin, C.-O.; Chane-Ching, K.M.; Majoral, J.-P. Cyclotriphosphazene, an old compound applied to the synthesis of smart dendrimers with tailored properties. Pure Appl. Chem. 2016, 88, 919–929. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Hameau, A.; Majoral, J.-P. The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans. 2016, 45, 1810–1822. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.-X.; Shi, X.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P. Cyclotriphosphazene core-based dendrimers for biomedical applications: An update on recent advances. J. Mater. Chem. B 2018, 6, 884–895. [Google Scholar] [CrossRef]
- de Jong, E.R.; Deloch, N.; Knoll, W.; Turrin, C.O.; Majoral, J.P.; Caminade, A.M.; Koper, I. Synthesis and characterization of bifunctional dendrimers: Preliminary use for the coating of gold surfaces and the proliferation of human osteoblasts (HOB). New J. Chem. 2015, 39, 7194–7205. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Fan, Y.; Qiu, J.; Laurent, R.; Li, J.; Bignon, J.; Mignani, S.; Caminade, A.-M.; Shi, X.; Majoral, J.-P. Potent Anticancer Efficacy of First-In-Class CuII and AuIII Metaled Phosphorus Dendrons with Distinct Cell Death Pathways. Chem. Eur. J. 2020, 26, 5903–5910. [Google Scholar] [CrossRef] [PubMed]
- Haensler, J.; Szoka, F.C. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjugate Chem. 1993, 4, 372–379. [Google Scholar] [CrossRef]
- Kim, D.H.; Karan, P.; Goring, P.; Leclaire, J.; Caminade, A.M.; Majoral, J.P.; Gosele, U.; Steinhart, M.; Knoll, W. Formation of dendrimer nanotubes by layer-by-layer deposition. Small 2005, 1, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.L.; Zhong, X.H.; Steinhart, M.; Caminade, A.M.; Majoral, J.P.; Knoll, W. Functional quantum-dot/dendrimer nanotubes for sensitive detection of DNA hybridization. Small 2008, 4, 566–571. [Google Scholar] [CrossRef]
- Kim, B.S.; Lebedeva, O.V.; Kim, D.H.; Caminade, A.M.; Majoral, J.P.; Knoll, W.; Vinogradova, O.I. Assembly and mechanical properties of phosphorus dendrimer/polyelectrolyte multilayer microcapsules. Langmuir 2005, 21, 7200–7206. [Google Scholar] [CrossRef]
- Reinert, P.; Chane-Ching, J.Y.; Bull, L.; Dagiral, R.; Batail, P.; Laurent, R.; Caminade, A.M.; Majoral, J.P. Influence of cationic phosphorus dendrimers on the surfactant-induced synthesis of mesostructured nanoporous silica. New J. Chem. 2007, 31, 1259–1263. [Google Scholar] [CrossRef] [Green Version]
- Beraa, A.; Hajjaji, M.; Laurent, R.; Caminade, A.M. Dendrimers-containing organoclays: Characterisation and interaction with methylene blue. Appl. Clay Sci. 2017, 136, 142–151. [Google Scholar] [CrossRef]
- Loup, C.; Zanta, M.A.; Caminade, A.M.; Majoral, J.P.; Meunier, B. Preparation of water-soluble cationic phosphorus-containing dendrimers as DNA transfecting agents. Chem. Eur. J. 1999, 5, 3644–3650. [Google Scholar] [CrossRef]
- Tsien, R.Y. The green fluorescent protein. Ann. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef] [PubMed]
- Maszewska, M.; Leclaire, J.; Cieslak, M.; Nawrot, B.; Okruszek, A.; Caminade, A.M.; Majoral, J.P. Water-soluble polycationic dendrimers with a phosphoramidothioate backbone: Preliminary studies of cytotoxicity and oligonucleotide/plasmid delivery in human cell culture. Oligonucleotides 2003, 13, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Padie, C.; Maszewska, M.; Majchrzak, K.; Nawrot, B.; Caminade, A.M.; Majoral, J.P. Polycationic phosphorus dendrimers: Synthesis, characterization, study of cytotoxicity, complexation of DNA, and transfection experiments. New J. Chem. 2009, 33, 318–326. [Google Scholar] [CrossRef]
- Ionov, M.; Gardikis, K.; Wróbel, D.; Hatziantoniou, S.; Mourelatou, H.; Majoral, J.-P.; Klajnert, B.; Bryszewska, M.; Demetzos, C. Interaction of cationic phosphorus dendrimers (CPD) with charged and neutral lipid membranes. Colloids Surf. B: Biointerfaces 2011, 82, 8–12. [Google Scholar] [CrossRef]
- Shcharbin, D.; Dzmitruk, V.; Shakhbazau, A.; Goncharova, N.; Seviaryn, I.; Kosmacheva, S.; Potapnev, M.; Pedziwiatr-Werbicka, E.; Bryszewska, M.; Talabaev, M.; et al. Fourth generation phosphorus-containing dendrimers: Prospective drug and gene delivery carrier. Pharmaceutics 2011, 3, 458–473. [Google Scholar] [CrossRef] [Green Version]
- Ochsner, M. Photophysical and Photobiological Processes in the Photodynamic Therapy of Tumours. J. Photochem. Photobiol. B 1997, 39, 1–18. [Google Scholar] [CrossRef]
- Xu, D.; Neckers, D.C. Aggregation of Rose Bengal Molecules in Solution. J. Photochem. Photobiol. A 1987, 40, 361–370. [Google Scholar] [CrossRef]
- Dabrzalska, M.; Janaszewska, A.; Zablocka, M.; Mignani, S.; Majoral, J.-P.; Klajnert-Maculewicz, B. Cationic phosphorus dendrimer enhances photodynamic activity of rose bengal against basal cell carcinoma cell lines. Mol. Pharm. 2017, 14, 1821–1830. [Google Scholar] [CrossRef]
- Moy, J.M.; Qu, Z.; Cobb, C.E. Reduction and uptake of methylene blue by human erythrocytes. Am. J. Physiol. Cell Physiol. 2004, 286, 1390–1398. [Google Scholar] [CrossRef] [Green Version]
- Blanzat, M.; Turrin, C.O.; Aubertin, A.M.; Couturier-Vidal, C.; Caminade, A.M.; Majoral, J.P.; Rico-Lattes, I.; Lattes, A. Dendritic catanionic assemblies: In vitro anti-HIV activity of phosphorus-containing dendrimers bearing Gal beta(1)cer analogues. ChemBioChem 2005, 6, 2207–2213. [Google Scholar] [CrossRef]
- Dabrzalska, M.; Janaszewska, A.; Zablocka, M.; Mignani, S.; Majoral, J.P.; Klajnert-Maculewicz, B. Complexing Methylene Blue with Phosphorus Dendrimers to Increase Photodynamic Activity. Molecules 2017, 22, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Dzmitruk, V.; Szulc, A.; Shcharbin, D.; Janaszewska, A.; Shcharbina, N.; Lazniewska, J.; Novopashina, D.; Buyanova, M.; Ionov, M.; Klajnert-Maculewicz, B.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (B). Efficiency of pharmacological action. Int. J. Pharm. 2015, 485, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Law, J.H.; Fotovati, A.; Dunn, S.E. Small interfering RNA library screen identified polo-like kinase-1 (PLK1) as a potential therapeutic target for breast cancer that uniquely eliminates tumor initiating cells. Breast Cancer Res. 2012, 14, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Mahira, S.; Majoral, J.-P.; Bryszewska, M.; Khan, W.; Ionov, M. Dendrimer mediated targeting of siRNA against polo-like kinase for the treatment of triple negative breast cancer. J. Biomed. Mater. Res. 2019, 107, 1933–1944. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Yu, F.; Qiu, J.; Cao, L.; Laurent, R.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P.; Shi, X. Revisiting cationic phosphorus dendrimers as a nonviral vector for optimized gene delivery towards cancer therapy applications. Biomacromolecules 2020, 21, 2502–2511. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Brotin, E.; Meryet-Figuière, M.; Simonin, K.; Duval, R.E.; Villedieu, M.; Leroy-Dudal, J.; Saison-Behmoaras, E.; Gauduchon, P.; Denoyelle, C.; Poulain, L. Bcl-XL and MCL-1 constitute pertinent targets in ovarian carcinoma and their concomitant inhibition is sufficient to induce apoptosis. Int. J. Cancer 2010, 126, 885–895. [Google Scholar] [CrossRef]
- Ihnatsyeu-Kachan, A.; Dzmitruk, V.; Apartsin, E.; Krasheninina, O.; Ionov, M.; Loznikova, S.; Venyaminova, A.; Miłowska, K.; Shcharbin, D.; Mignani, S.; et al. Multi-target inhibition of cancer cell growth by siRNA cocktails and 5-fluorouracil using effective piperidine-terminated phosphorus dendrimers. Colloids Interfaces 2017, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Takahashi, R.; Muira, C.; Baba, Y. Synthetic Study of Peptide Aldehydes. Chem. Pharm. Bull. 1975, 23, 3106–3113. [Google Scholar] [CrossRef] [Green Version]
- Mignani, S.; El Brahmi, N.; Cresteil, T.; Majoral, J.-P. First-in-Class Combination Therapy of a Copper(II) Metallo-Phosphorus Dendrimer with Cytotoxic Agents. Oncology 2018, 94, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Varilh, M.; Turrin, C.O.; Saoudi, A.; Caminade, A.M.; Poupot, R.; Liblau, R.S. Phosphorus-Based Dendrimer ABP Treats Neuroinflammation by Promoting IL-10-Producing CD4(+) T Cells. Biomacromolecules 2015, 16, 3425–3433. [Google Scholar] [CrossRef]
- Jebbawi, R.; Oukhrib, A.; Clement, E.; Blanzat, M.; Turrin, C.O.; Caminade, A.M.; Lacoste, E.; Fruchon, S.; Poupot, R. An Anti-Inflammatory Poly(PhosphorHydrazone) Dendrimer Capped with AzaBisPhosphonate Groups to Treat Psoriasis. Biomolecules 2020, 10, 949. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Griffe, L.; Poupot, M.; Marchand, P.; Maraval, A.; Turrin, C.-O.; Rolland, O.; Métivier, P.; Bacquet, G.; Fournié, J.-J.; Caminade, A.-M.; et al. Multiplication of Human Natural Killer Cells by Nanosized Phosphonate-Capped Dendrimers. Angew. Chem. Int. Ed. 2007, 46, 2523–2526. [Google Scholar] [CrossRef]
- Poupot, M.; Griffe, L.; Marchand, P.; Maraval, A.; Rolland, O.; Martinet, L.; L’Faqihi-Olive, F.E.; Turrin, C.-O.; Caminade, A.-M.; Fournie, J.-J.; et al. Design of phosphorylated dendritic architectures to promote human monocyte activation. FASEB J. 2006, 20, 2339–2351. [Google Scholar] [CrossRef]
- Portevin, D.; Poupot, M.; Rolland, O.; Turrin, C.-O.; Fournié, J.-J.; Majoral, J.-P.; Caminade, A.-M.; Poupot, R. Regulatory activity of azabisphosphonate-capped dendrimers on human CD4+ T cell proliferation for ex-vivo expansion of NK cells from PBMCs and immunotherapy. J. Trans. Med. 2009, 7, 82. [Google Scholar] [CrossRef]
- Poupot, M.; Turrin, C.-O.; Caminade, A.-M.; Fournie, J.-J.; Attal, M.; Poupot, R.; Fruchon, S. Poly(phosphorhydrazone) dendrimers: Yin and yang of monocyte activation for human NK cell amplification applied to immunotherapy against Multiple Myeloma. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2321–2330. [Google Scholar] [CrossRef]
- Caminade, A.M.; Fruchon, S.; Turrin, C.O.; Poupot, M.; Ouali, A.; Maraval, A.; Garzoni, M.; Maly, M.; Furer, V.; Kovalenko, V.; et al. The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nat. Comm. 2015, 6, 7722. [Google Scholar] [CrossRef]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mongin, O.; Rama Krishna, T.; Werts, M.H.V.; Caminade, A.-M.; Majoral, J.P.; Blanchard-Desce, M. A modular approach to two-photon absorbing organic nanodots: Brilliant dendrimers as an alternative to semiconductor quantum dots? Chem. Commun. 2006, 915–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terenziani, F.; Parthasarathy, V.; Pla-Quintana, A.; Maishal, T.; Caminade, A.M.; Majoral, J.-P.; Blanchard-Desce, M. Cooperative two-photon absorption enhancement via through-space interactions in covalent multichromophoric nanoassemblies. Angew. Chem. Int. Ed. 2009, 48, 8691–8694. [Google Scholar] [CrossRef]
- Krishna, T.R.; Parent, M.; Werts, M.H.V.; Moreaux, L.; Gmouh, S.; Charpak, S.; Caminade, A.-M.; Majoral, J.-P.; Blanchard-Desce, M. Water-soluble dendrimeric two-photon markers for in vivo imaging. Angew. Chem. Int. Ed. 2006, 45, 4645–4648. [Google Scholar] [CrossRef] [PubMed]
- Sourdon, A.; Gary-Bobo, M.; Maynadier, M.; Garcia, M.; Majoral, J.-P.; Caminade, A.-M.; Mongin, O.; Blanchard-Desce, M. Dendrimeric nanoparticles for two-photon photodynamic therapy and imaging: Synthesis, photophysical properties, innocuousness in daylight and cytotoxicity under two-photon irradiation in the NIR. Chem. Eur. J. 2019, 25, 3637–3649. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Caminade, A.M.; Laurent, R.; Shi, X.Y.; Majoral, J.P. Recent therapeutic applications of the theranostic principle with dendrimers in oncology. Sci. China Mater. 2018, 61, 1367–1386. [Google Scholar] [CrossRef] [Green Version]
- Starpharma. Available online: https://starpharma.com/vivagel (accessed on 17 July 2020).
- Starpharma. Available online: https://starpharma.com/drug_delivery (accessed on 17 July 2020).















| Dendrimer | KB | HL-60 | EPC | EPC/KB | EPC/HL-60 |
|---|---|---|---|---|---|
| 4a-G3 | 1600 ± 150 | 1300 ± 100 | 360 ± 200 | 0.225 | 0.277 |
| 4a-Cu-G3 | 470 ± 20 | 580 ± 70 | 800 ± 180 | 1.7 | 1.4 |
| 4a-Au-G3 | 7.5 ± 7.5 | 3.3 ± 0.6 | >1000 | >133 | >303 |
| 4a-G3-[NN44PEG4] | >1000 | >1000 | >1000 | ||
| 4a-G3-[NN40PEG8] | >1000 | >1000 | >1000 | ||
| 4a-G3-[NN30PEG18] | >1000 | >1000 | >1000 | ||
| 4a-G3-[Au2x15NN15PEG18] | 87 ± 7 | >1000 | >1000 | >11.5 | |
| 4a-G3-[Au2x20NN20PEG8] | 15 ± 5 | 4.5 ± 0.5 | >1000 | >67 | >222 |
| 4a-G3-[Au2x22NN22PEG4] | 6.7 ± 4.6 | 3 ± 0.5 | >1000 | >149 | >333 |
| 4a-G3-[Au2x20Cu20PEG8] | 8.5 ± 0.7 | 2.5 ± 0.7 | >1000 | >118 | >400 |
| 4a-G3-[Au2x10Cu20NN10PEG8] | 10 ± 3 | 4 ± 3 | >1000 | >100 | >250 |
| 4a-G3-[Au2x40PEG8] | 5.5 ± 0.5 | 1.7 ± 0.5 | >1000 | >182 | >588 |
| 4a-Fe-G3 | >1000 | >1000 | >1000 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caminade, A.-M. Phosphorus Dendrimers as Nanotools against Cancers. Molecules 2020, 25, 3333. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25153333
Caminade A-M. Phosphorus Dendrimers as Nanotools against Cancers. Molecules. 2020; 25(15):3333. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25153333
Chicago/Turabian StyleCaminade, Anne-Marie. 2020. "Phosphorus Dendrimers as Nanotools against Cancers" Molecules 25, no. 15: 3333. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25153333