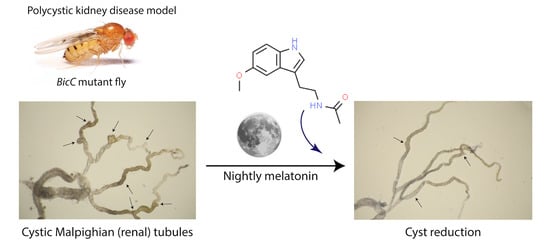
Cyst Reduction by Melatonin in a Novel Drosophila Model of Polycystic Kidney Disease
Abstract
:1. Introduction
1.1. Polycystic Kidney Disease
1.2. Melatonin
2. Results
2.1. Melatonin Significantly Reduced Cysts in the Renal Tubule of BicCΔ/YC33 Mutants
2.2. Melatonin Treatment Displayed Regional Specificity in BicCΔ/YC33 Mutants
2.3. Melatonin Treatment Was Less Efficient in BicCΔ/IIF34 Mutants
3. Discussion
4. Materials and Methods
4.1. Fly Lines and Husbandry
4.2. Cystic Index
4.3. Microscopy
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 50. [Google Scholar] [CrossRef]
- Rowe, I.; Chiaravalli, M.; Mannella, V.; Ulisse, V.; Quilici, G.; Pema, M.; Song, X.W.; Xu, H.; Mari, S.; Qian, F.; et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 2013, 19, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.J. Polycystic kidney disease: Neoplasia in disguise. Am. J. Kidney Dis. 1990, 15, 110–116. [Google Scholar] [CrossRef]
- Harris, P.C.; Watson, M.L. Autosomal dominant polycystic kidney disease: Neoplasia in disguise? Nephrol. Dial. Transplant. 1997, 12, 1089–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, T.; Pelling, J.C.; Ramaswamy, N.T.; Eppler, J.W.; Wallace, D.P.; Nagao, S.; Rome, L.A.; Sullivan, L.P.; Grantham, J.J. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int. 2000, 57, 1460–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shillingford, J.M.; Murci, N.S.; Larson, C.H.; Low, S.H.; Hedgepeth, R.; Brown, N.; Flask, C.A.; Novick, A.C.; Goldfarb, D.A.; Kramer-Zucker, A.; et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5466–5471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, S.J.; Amsler, K.; Hyink, D.P.; Li, X.; Lu, W.; Zhou, J.; Burrow, C.R.; Wilson, P.D. Inhibition of HER-2 (new/ErbB2) restores normal function and structure to polycystic kidney disease (PKD) epithelia. Biochim. Biophys. Acta 2006, 1762, 647–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, W.E.; von Vigier, R.O.; Frost, P.; Avner, E.D. Src inhibition ameliorates polycystic kidney disease. J. Am. Soc. Nephrol. 2008, 19, 1331–1341. [Google Scholar] [CrossRef] [Green Version]
- Lancaster, M.A.; Louie, C.M.; Silhavy, J.L.; Sintasath, L.; DeCambre, M.; Nigam, S.K.; Willert, K.; Gleeson, J.G. Impaired Wnt-β-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat. Med. 2009, 15, 1046–1054. [Google Scholar] [CrossRef] [Green Version]
- Shillingford, J.M.; Piontek, K.B.; Germino, G.G.; Weimbs, T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J. Am. Soc. Nephrol. 2010, 21, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Varelas, X.; Miller, B.W.; Sopko, R.; Song, S.; Gregorieff, A.; Fellouse, F.A.; Sakuma, R.; Pawson, T.; Hunziker, W.; McNeill, H.; et al. The Hippo pathway regulates Wnt/β-catenin signaling. Dev. Cell 2010, 18, 579–591. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Reif, G.A.; Calvet, J.P.; Wallace, D.P. Sorafenib inhibits cAMP-dependent ERK activation, cell proliferation, and in vitro cyst growth of human ADPKD cyst epithelial cells. Am. J. Physiol. Renal Phys. 2010, 299, F944–F951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, P.D. Apico-basal polarity in polycystic kidney disease epithelia. Biochim. Biophys. Acta 2011, 1812, 1239–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedeles, S.; Gallagher, A.R. Cell polarity and cystic kidney disease. Pediatr. Nephrol. 2013, 28, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Goggolidou, P. Wnt and planar cell polarity signaling in cystic renal disease. Organogenesis 2014, 10, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeger-Nukpezah, T.; Geynisman, D.M.; Nikonova, A.S.; Benzing, T.; Golemis, E.A. The hallmarks of cancer: Relevance to the pathogenesis of polycystic kidney disease. Nat. Rev. Nephrol. 2015, 11, 515–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, C.S.; Aldred, M.; von Ruhland, C.; Harris, R.; Sandford, R.; Cheadle, J.P. Defects in cell polarity underlie TSC and ADPKD-associated cystogenesis. Hum. Mol. Genet. 2009, 18, 2166–2176. [Google Scholar] [CrossRef]
- Fischer, D.C.; Jacoby, U.; Pape, L.; Ward, C.J.; Kuwertz-Broeking, E.; Renken, C.; Nizze, H.; Querfeld, U.; Rudolph, B.; Mueller-Wiefel, D.E.; et al. Activation of the AKT/mTOR pathway in autosomal recessive polycystic kidney disease (ARPKD). Nephrol. Dial. Transplant. 2009, 24, 1819–1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, T.R.; Liu, D.; Zilfou, J.T.; Robb, V.; Morrison, T.; Watnick, T.; Henske, E.P. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum. Mol. Genet. 2009, 18, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Ibraghimov-Beskrovnaya, O.; Natoli, T.A. mTOR signaling in polycystic kidney disease. Trends Mol. Med. 2011, 17, 625–633. [Google Scholar] [CrossRef]
- Gamberi, C.; Hipfner, D.R.; Trudel, M.; Lubell, W.D. Bicaudal C mutation causes myc and TOR pathway up-regulation and polycystic kidney disease-like phenotypes in Drosophila. PLoS Genet. 2017, 13, e1006694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanoix, J.; D’Agati, V.; Szabolcs, M.; Trudel, M. Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD). Oncogene 1996, 13, 1153–1160. [Google Scholar] [PubMed]
- Trudel, M. C-Myc signalling in the genetic mechanism of polycystic kidney disease. In Polycystic Kidney Disease; Li, X., Ed.; Codon Publications: Brisbane, Australia, 2015; pp. 231–257. [Google Scholar]
- Happe, H.; Peters, J.D.M. Translational research in ADPKD: Lessons from animal models. Nat. Rev. Nephrol. 2014, 10, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Gamberi, C.; Lasko, P. The Bic-C family of developmental translational regulators. Int. J. Genom. 2012, 2012, 141386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, S.; Reiter, L.T.; Bier, E.; Gribskov, M. Homophila: Human disease gene cognates in Drosophila. Nucleic Acids Res. 2002, 30, 149–151. [Google Scholar] [CrossRef]
- Millet-Boureima, C.; Porras, J.M.; Gamberi, C. Modeling renal disease “on the fly”. BioMed Res. Int. 2018, 2018, 5697436. [Google Scholar] [CrossRef] [Green Version]
- Cohen, E.; Sawyer, J.K.; Peterson, N.G.; Dow, J.A.T.; Fox, D.T. Physiology, development, and disease modeling in the Drosophila excretory system. Genetics 2020, 214, 235–264. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Kean, L.; Yang, J.; Allan, A.K.; Davies, S.A.; Herzyk, P.; Dow, J.A.T. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biol. 2004, 5, R69. [Google Scholar] [CrossRef] [Green Version]
- Millet-Boureima, C.; Chingle, R.; Lubell, W.D.; Gamberi, C. Cyst reduction in a polycystic kidney disease Drosophila model using Smac mimics. Biomedicines 2019, 7, 82. [Google Scholar] [CrossRef] [Green Version]
- Millet-Boureima, C.; Selber-Hnatiw, S.; Gamberi, C. Drug discovery and chemical probing in Drosophila. Genome 2020, 999, 1–13. [Google Scholar] [CrossRef]
- Fan, L.X.; Zhou, X.; Sweeney, W.E.; Wallace, D.P.; Avner, E.D.; Grantham, J.J.; Li, X. Smac-mimetic-induced epithelial cell death reduces the growth of renal cysts. J. Am. Soc. Nephrol. 2013, 24, 2010–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sans-Atxer, L.; Joly, D. Tolvaptan in the treatment of autosomal dominant polycystic kidney disease: Patient selection and special considerations. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Chiaravalli, M.; Rowe, I.; Mannella, V.; Quilici, G.; Canu, T.; Bianchi, V.; Gurgone, A.; Antunes, S.; D’Adamo, P.; Esposito, A.; et al. 2-Deoxy-d-glucose ameliorates PKD progression. Am. J. Soc. Nephrol. 2016, 27, 1958–1969. [Google Scholar] [CrossRef] [Green Version]
- Kipp, K.R.; Rezaei, M.; Lin, L.; Dewey, E.C.; Weimbs, T. A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease. Am. J. Phys. Renal Phys. 2016, 310, F726–F731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, J.A.; Kruger, S.L.; Broderick, C.; Amarlkhagva, T.; Agrawal, S.; Dodam, J.R.; Mrug, M.; Lyons, L.A.; Weimbs, T. Ketosis ameliorates renal cyst growth in polycystic kidney disease. Cell Metab. 2019, 30, 1007–1023. [Google Scholar] [CrossRef]
- Hardeland, R.; Balzer, I.; Poeggeler, B.; Fuhrberg, B.; Una, H.; Behrmann, G.; Wolf, R.; Meyer, T.J.; Reiter, R.J. On the primary functions of melatonin in evolution: Mediation of photoperiodic signals in a unicell, photooxidation, and scavenging of free radicals. J. Pineal Res. 1995, 18, 104–111. [Google Scholar] [CrossRef]
- Venegas, C.; Garcia, J.A.; Escames, G.; Ortiz, F.; Lopez, A.; Doerrier, C.; Garcia-Corzo, L.; Lopez, L.C.; Reiter, R.J.; Acuna-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. 2008, 19, 17–24. [Google Scholar] [CrossRef]
- Bubenik, G.A. Localization of melatonin in the digestive tract of the rat. Effect of maturation, diurnal variation, melatonin treatment and pinealectomy. Horm. Res. 1980, 12, 313–323. [Google Scholar] [CrossRef]
- Lardone, P.J.; Guerrero, J.M.; Fernandez-Santos, J.M.; Rubio, A.; Martin-Lacave, I.; Carrillo-Vico, A. Melatonin synthesized by T lymphocytes as a ligand of the retinoic acid-related orphan receptor. J. Pineal Res. 2011, 51, 454–462. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [Green Version]
- Cipolla-Neto, J.; Gaspar do Amaral, F. Melatonin as a hormone: New physiological and clinical insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Tan, D.X.; Fuentes-Broto, L. Melatonin: A multitasking molecule. Prog. Brain Res. 2010, 181, 127–151. [Google Scholar]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Jenwitheesuk, A.; Nopparat, C.; Mukda, S.; Wongshitrat, P.; Govitrapong, P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int. J. Mol. Sci. 2014, 15, 16848–16884. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Hsieh, M.J.; Yang, W.E.; Chung, W.H.; Reiter, R.J.; Yang, S.F. Cancer metastasis: Mechanisms of inhibition by melatonin. J. Pineal Res. 2017, 62, e12370. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Patel, K.K.; Dehari, D.; Agrawal, A.K.; Singh, S. Melatonin and its ubiquitous anticancer effects. Mol. Cell. Biochem. 2019, 462, 133–155. [Google Scholar] [CrossRef]
- Sainz, R.M.; Mayo, J.C.; Rodriguez, C.; Tan, D.X.; Lopez-Burillo, S.; Reiter, R.J. Melatonin and cell death: Differential actions on apoptosis in normal and cancer cells. Cell. Mol. Life Sci. 2003, 60, 1407–1426. [Google Scholar] [CrossRef]
- Yousaf, F.; Seet, E.; Venkatraghavan, L.; Abrishami, A.; Chung, F. Efficacy and safety of melatonin as an anxiolytic and analgesic in the perioperative period: A qualitative systemic review of randomized trials. Anesthesiology 2010, 113, 968–976. [Google Scholar] [CrossRef] [Green Version]
- Pourhanifeh, M.H.; Sharifi, M.; Reiter, R.J.; Davoodabadi, A.; Asemi, Z. Melatonin and non-small cell lung cancer: New insights into signaling pathways. Cancer Cell Int. 2019, 19, 131. [Google Scholar] [CrossRef]
- Mantovani, M.; Kaster, M.P.; Pertile, R.; Calixto, J.B.; Rodrigues, A.L.S.; Santos, A.R.S. Mechanisms involved in the antinociception caused by melatonin in mice. J. Pineal Res. 2006, 41, 382–389. [Google Scholar] [CrossRef]
- Dilman, V.M.; Anisimov, V.N.; Ostroumova, M.N.; Khavinson, V.K.; Morozov, V.G. Increase in lifespan of rats following polypeptide pineal extract treatment. Exp. Pathol. (Jena) 1979, 17, 539–545. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Mylnikov, S.V.; Oparina, T.I.; Khavinson, V.K. Effect of melatonin and pineal peptide preparation epithalamin on life span and free radical oxidation in Drosophila melanogaster. Mech. Ageing Dev. 1997, 97, 81–91. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Zavarzina, N.Y.; Zabezhinski, M.A.; Popovich, I.G.; Zimina, O.A.; Shtylick, A.V.; Arutjunyan, A.V.; Oparina, T.I.; Prokopenko, V.M.; Mikhalski, A.I.; et al. Melatonin increases both life span and tumor incidence in female CBA mice. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B311–B323. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J. Mechanisms of cancer inhibition by melatonin. J. Pineal Res. 2004, 37, 213–214. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Popovich, I.G.; Zabezhinski, M.A.; Anisimov, S.V.; Vesnushkin, G.M.; Vinogradova, I.A. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim. Biophys. Acta 2006, 1757, 573–589. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.J.; Xu, D.P.; Li, H.B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.F.; Xu, K. Melatonin, a full service and anti-cancer agent: Inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef]
- Aubert, C.H.; Janiaud, P.; Lecalvez, J. Effect of pinealectomy and melatonin on mammary tumor growth in Sprague-Dawley rats under different conditions of lighting. J. Neural Transm. 1980, 47, 121–130. [Google Scholar] [CrossRef]
- Tamarkin, L.; Cohen, M.; Roselle, D.; Reichert, C.; Lippman, M.; Chabner, B. Melatonin inhibition and pinealectomy enhancement of 7, 12-dimethylbenz (a) anthracene-induced mammary tumors in the rat. Am. Assoc. Cancer Res. 1981, 41, 4432–4436. [Google Scholar]
- Mediavilla, M.D.; Cos, S.; Sanchez-Barcelo, E.J. Melatonin increases p53 and p21 WAF1 expression in MCF-7 human breast cancer cells in vitro. Life Sci. 1999, 65, 415–420. [Google Scholar] [CrossRef]
- Cos, S.; Mediavilla, M.D.; Fernandez, R.; Gonzalez-Lamuno, D.; Sanchez-Barcelo, E.J. Does melatonin induce apoptosis in MCF-7 human breast cancer cells in vitro? J. Pineal Res. 2002, 32, 90–96. [Google Scholar] [CrossRef]
- Cos, S.; Martinez-Campa, C.; Mediavilla, M.D.; Sanchez-Barcelo, E.J. Melatonin modulates aromatase activity in MCF-7 human breast cancer cells. J. Pineal Res. 2005, 38, 136–142. [Google Scholar] [CrossRef]
- Martinez-Campa, C.; Alonso-Gonzalez, C.; Mediavilla, M.D.; Cos, S.; Gonzalez, A.; Ramos, S.; Sanchez-Barcelo, E.J. Melatonin inhibits both Erα activation and breast cancer cell proliferation induced by a metalloestrogen, cadmium. J. Pineal Res. 2006, 40, 291–296. [Google Scholar] [CrossRef]
- Hill, S.M.; Belancio, V.P.; Dauchy, R.T.; Xiang, S.; Brimer, S.; Mao, L.; Hauch, A.; Lundberg, P.W.; Summers, W.; Yuan, L.; et al. Melatonin: An inhibitor of breast cancer. Endocr. Relat. Cancer 2015, 22, R183–R204. [Google Scholar] [CrossRef] [Green Version]
- Fischer, T.W.; Zmijewski, M.A.; Zbytek, B.; Sweatman, T.W.; Slominski, R.M.; Wortsman, J.; Slominski, A. Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int. J. Oncol. 2006, 29, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, J.; Negrin, G.; Estevez, F.; Loro, J.; Reiter, R.J.; Quintana, J. Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J. Pineal Res. 2010, 49, 45–54. [Google Scholar] [CrossRef]
- Choi, S.I.; Kim, K.S.; Oh, J.Y.; Jin, J.Y.; Lee, G.H.; Kim, E.K. Melatonin induces autophagy via an mTOR-dependent pathway and enhances clearance of mutant-TGFBlp. J. Pineal Res. 2013, 54, 361–372. [Google Scholar] [CrossRef]
- Ding, K.; Wang, H.; Xu, J.; Lu, X.; Zhang, L.; Zhu, L. Melatonin reduced microglial activation and alleviated neuroinflammation induced neuron degeneration in experimental traumatic brain injury: Possible involvement of mTOR pathway. Neurochem. Int. 2014, 76, 23–31. [Google Scholar] [CrossRef]
- Kang, J.W.; Cho, H.I.; Lee, S.M. Melatonin inhibits mTOR-dependent autophagy during liver ischemia/reperfusion. Cell. Physiol. Biochem. 2014, 33, 23–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Kim, T.J.; Yoo, Y.M. Melatonin combined with endoplasmic reticulum stress induces cell death via the PI3K/Akt/mTOR pathway in B16F10 melanoma cells. PLoS ONE 2014, 9, e92627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Fan, M.; Chen, Y.; Zhao, Q.; Song, C.; Yan, Y.; Jin, Y.; Huang, Z.; Lin, C.; Wu, J. Melatonin induces cell apoptosis and AGS cells through the activation of JNK and P38 MAPK and the suppression of nuclear factor-kappa B: A novel therapeutic implication for gastric cancer. Cell. Physiol. Biochem. 2015, 37, 2323–2338. [Google Scholar] [CrossRef]
- Behram Kandemir, Y.; Aydin, C.; Gorgisen, G. The effects of melatonin on oxidative stress and prevention of primordial follicle loss via activation of mTOR pathway in the rat ovary. Cell. Mol. Biol. (Noisy-Le-Grand) 2017, 63, 100–106. [Google Scholar] [CrossRef]
- Tan, D.X.; Chen, L.D.; Peoggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Reiter, R.J. Oxidative damage to nuclear DNA: Amelioration by melatonin. Neuro. Endocrinol. Lett. 1999, 20, 145–150. [Google Scholar]
- Karbownik, M.; Tan, D.X.; Reiter, R.J. Melatonin reduces the oxidation of nuclear DNA and membrane lipids induced by the carcinogen δ-aminolevulinic acid. Int. J. Cancer 2000, 88, 7–11. [Google Scholar] [CrossRef]
- Khavinson, V.K.; Izmaylov, D.M.; Obukhova, L.K.; Malinin, V.V. Effect of epitalon on the lifespan increase in Drosophila melanogaster. Mech. Ageing Dev. 2000, 120, 141–149. [Google Scholar] [CrossRef]
- Qi, W.; Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Siu, A.W.; Garcia, J.J. Increased levels of oxidatively damaged DNA induced by chromium (III) and H2O2: Protection by melatonin and related molecules. J. Pineal Res. 2000, 29, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Melatonin: Lowering the high price of free radicals. News Physiol. Sci. 2000, 15, 246–250. [Google Scholar] [CrossRef]
- Karbownik, M.; Lewinski, A.; Reiter, R.J. Anticarcinogenic actions of melatonin which involve antioxidative processes: Comparison with other antioxidants. Int. J. Biochem. Cell Biol. 2001, 33, 735–753. [Google Scholar] [CrossRef]
- Karbownik, M.; Reiter, R.J. Melatonin protects against oxidative stress caused by d-aminolevulinic acid: Implications for cancer. Cancer Investig. 2002, 20, 276–286. [Google Scholar] [CrossRef]
- Yang, Q.H.; Xu, J.N.; Xu, R.K.; Pang, S.F. Inhibitory effects of melatonin on the growth of pituitary prolactin-secreting tumor in rats. J. Pineal Res. 2006, 40, 230–235. [Google Scholar] [CrossRef]
- Rossi, S.P.; Windschuetti, S.; Matzkin, M.E.; Terradas, C.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Calandra, R.S.; Mayerhofer, A.; Frungieri, M.B. Melatonin in testes of infertile men: Evidence for anti-proliferative and anti-oxidant effects on local macrophage and mast cell populations. Andrology 2014, 2, 436–449. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Burattini, S.; Battistelli, M.; Codenotti, S.; Falcieri, E.; Fanzani, A.; Salucci, S. Melatonin action in tumor skeletal muscle cells: An ultrastructural study. Acta Histochem. 2016, 118, 278–285. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [Green Version]
- Longaretti, L.M.; Luciano, J.A.; Strapazzon, G.; Pereira, M.; Damiani, A.P.; Rohr, P.; Rigo, F.K.; Alves de Oliveira, C.; Steiner, B.T.; Vilela, T.C.; et al. Anti-genotoxic and anti-mutagenic effects of melatonin supplementation in a mouse model of melanoma. Drug Chem. Toxicol. 2020, 1–8. [Google Scholar] [CrossRef]
- Leon-Blanco, M.M.; Guerrero, J.M.; Reiter, R.J.; Calvo, J.R.; Pozo, D. Melatonin inhibits telomerase activity in the MCF-7 tumor cell line both in vivo and in vitro. J. Pineal Res. 2003, 35, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Leja-Szpak, A.; Jaworek, J.; Pierzchalski, P.; Reiter, R.J. Melatonin induces pro-apoptotic signaling pathway in human pancreatic carcinoma cells (PANC-1). J. Pineal Res. 2010, 49, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Proietti, S.; Cucina, A.; Reiter, R.J. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: A review. Expert Opin. Ther. Targets 2013, 17, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Spence, D.W.; Pandi-Perumal, S.R.; Trakht, I.; Cardinalli, D.P. Therapeutic actions of melatonin in cancer: Possible mechanisms. Integr. Cancer Ther. 2008, 7, 189–203. [Google Scholar] [CrossRef]
- Lv, D.; Cui, P.L.; Yao, S.W.; Xu, Y.Q.; Yang, Z.X. Melatonin inhibits the expression of vascular endothelial growth factor in pancreatic cancer cells. Chin. J. Cancer Res. 2012, 24, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Garcia, V.; Gonzalez, A.; Alonso-Gonzalez, C.; Martinez-Campa, C.; Cos, S. Antiangiogenic effects of melatonin in endothelial cell cultures. Microvasc. Res. 2013, 87, 25–33. [Google Scholar] [CrossRef]
- Ma, Q.; Reiter, R.J.; Chen, Y. Role of melatonin in controlling angiogenesis under physiological and pathological conditions. Angiogenesis 2020, 23, 91–104. [Google Scholar] [CrossRef]
- Parker, M.I.; Nikonova, A.S.; Sun, D.; Golemis, E.A. Proliferative signaling by ERBB proteins and RAF/MEK/ERK effectors in polycystic kidney disease. Cell. Signal. 2020, 67, 109497. [Google Scholar] [CrossRef]
- Maser, R.L.; Vassmer, D.; Magenheimer, B.S.; Clavet, J.P. Oxidant stress and reduced antioxidant enzyme protection in polycystic kidney disease. J. Am. Soc. Nephrol. 2002, 13, 991–999. [Google Scholar]
- Menon, V.; Rudym, D.; Chandra, P.; Miskulin, D.; Perrone, R.; Sarnak, M. Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Klawitter, J.; Reed-Gitomer, B.Y.; McFann, K.; Pennington, A.; Klawitter, J.; Abebe, K.Z.; Klepacki, J.; Cadnapaphornchai, M.A.; Brosnahan, G.; Chonchoi, M.; et al. Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2014, 307, F1198–F1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrandon, D.; Imler, J.L.; Hetru, C.; Hoffmann, J.A. The Drosophila systemic immune response: Sensing and signaling during bacterial and fungal infections. Nat. Rev. Immunol. 2007, 7, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Broderick, N.A.; Chakrabarti, S.; Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009, 23, 2333–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.C.; Liao, C.W.; Pan, R.L.; Juang, J.L. Infection-induced intestinal oxidative stress triggers organ-to-organ immunological communication in Drosophila. Cell Host Microbe 2012, 11, 410–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahnke, G.; Marr, M.; Myers, C.; Wilson, R.; Travlos, G.; Price, C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci. 1999, 50, 271–279. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Gogenur, I.; Rosenberg, J.; Reiter, R.J. The safety of melatonin in humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Saffman, E.E.; Styhler, S.; Rother, K.; Li, W.; Richard, S.; Lasko, P. Premature translation of oskar in oocytes lacking the RNA-binding protein bicaudal-C. Mol. Cell. Biol. 1998, 18, 4855–4862. [Google Scholar] [CrossRef] [Green Version]
- Quiroz, Y.; Ferrebuz, A.; Romero, F.; Vaziri, N.D.; Rodriguez-Iturbe, B. Melatonin ameliorates oxidative stress, inflammation, proteinuria, and progression of renal damage in rats with renal mass reduction. Am. J. Physiol. Renal Physiol. 2008, 294, F336–F344. [Google Scholar] [CrossRef]
- Koch, B.C.P.; van der Putten, K.; Van Someren, E.J.W.; Wielders, J.P.M.; Ter Wee, P.M.; Nagtegaal, J.E.; Gaillard, C.A.J.M. Impairment of endogenous melatonin rhythm is related to the degree of chronic kidney disease (CREAM study). Nephrol. Dial. Transplant. 2010, 25, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Russcher, M.; Koch, B.; Nagtegaal, E.; van der Putten, K.; ter Wee, P.; Gaillard, C. The role of melatonin treatment in chronic kidney disease. Front. Biosci. 2012, 17, 2644–2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, N.; Ishigaki, S.; Isobe, S. The pivotal role of melatonin in ameliorating chronic kidney disease by suppression of the renin-angiotensin system in the kidney. Hypertens. Res. 2019, 42, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Oktem, F.; Ozguner, F.; Mollaoglu, H.; Koyu, A.; Uz, E. Oxidative damage in the kidney induced by 900-MHz-emitted mobile phone: Protection by melatonin. Arc. Med. Res. 2005, 36, 350–355. [Google Scholar] [CrossRef]
- Adewole, S.O.; Salako, A.A.; Doherty, O.W.; Naicker, T. Effect of melatonin on carbon tetrachloride-induced kidney injury in Wistar rats. Afr. J. Biomed. Res. 2007, 10, 153–164. [Google Scholar] [CrossRef]
- Mahieu, S.; del Carmen Contini, M.; Gonzalez, M.; Millen, N. Melatonin reduces oxidative damage induced by aluminium in rat kidney. Toxicol. Lett. 2009, 190, 9–15. [Google Scholar] [CrossRef]
- Kobori, H.; Katsurada, A.; Ozawa, Y.; Satou, R.; Miyata, K.; Hase, N.; Suzaki, Y.; Shoji, T. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem. Biophys. Res. Commun. 2007, 358, 156–163. [Google Scholar]
- Kamiyama, M.; Urushihara, M.; Morikawa, T.; Konishi, Y.; Imanishi, M.; Nishiyama, A.; Kobori, H. Oxidative stress/angiotensinogen/renin-angiotensin system axis in patients with diabetic nephropathy. Int. J. Mol. Sci. 2013, 14, 23045–23062. [Google Scholar] [CrossRef] [Green Version]
- Andries, A.; Daenen, K.; Jouret, F.; Bammens, B.; Mekahli, D.; Van Schepdael, A. Oxidative stress in autosomal dominant polycystic kidney disease: Player and/or early predictor for disease progression? Pediatr. Nephrol. 2019, 34, 993–1008. [Google Scholar] [CrossRef] [Green Version]
- Toung, Y.P.; Hsieh, T.S.; Tu, C.P. The glutathione S-transferase D genes. A divergently organized, intronless gene family in Drosophila melanogaster. J. Biol. Chem. 1993, 268, 9737–9746. [Google Scholar]
- Kabil, H.; Partridge, L.; Harshman, L.G. Superoxide dismutase activities in long-lived Drosophila melanogaster females: Chico1 genotypes and dietary dilution. Biogenrontology 2007, 8, 201–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, I.; Kim, T.Y.; Kim-Ha, J. Identification of Drosophila SOD3 and its protective role against phototoxic damage to cells. FEBS Lett. 2011, 585, 1973–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhagen, A.M.; Vaux, D.L. Cell death regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis 2002, 7, 163–166. [Google Scholar] [CrossRef]
- Lalaoui, N.; Vaux, D.L. Recent advances in understanding inhibitor of apoptosis proteins. F1000Research 2018, 7, F1000 Faculty Rev-1889. [Google Scholar] [CrossRef]
- Cong, H.; Xu, L.; Wu, Y.; Qu, Z.; Bian, T.; Zhang, W.; Xing, C.; Zhuang, C. Inhibitor of apoptosis protein (IAP) antagonists in anticancer agent discovery: Current status and perspectives. J. Med. Chem. 2019, 62, 5750–5772. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Magenheimer, B.S.; Xia, S.; Johnson, T.; Wallace, D.P.; Calvet, J.P.; Li, R. A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat. Med. 2008, 14, 863–868. [Google Scholar] [CrossRef] [Green Version]
- Igaki, T.; Kanda, H.; Yamamoto-Goto, Y.; Kanuka, H.; Kuranaga, E.; Aigaki, T.; Miura, M. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J. 2002, 21, 3009–3018. [Google Scholar] [CrossRef]
- Kanda, H.; Igaki, T.; Kanuka, H.; Yagi, T.; Miura, M. Wengen, a member of the Drosophila tumor necrosis factor receptor superfamily, is required for Eiger signaling. J. Biol. Chem. 2002, 277, 28372–28375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, E.; Yan, M.; Basler, K. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr. Biol. 2002, 12, 1263–1268. [Google Scholar] [CrossRef] [Green Version]
- Andersen, D.S.; Colombani, J.; Palmerini, V.; Chakrabandhu, K.; Boone, E.; Rothlisberger, M.; Toggweiler, J.; Basler, J.; Mapelli, M.; Hueber, A.O.; et al. The Drosophila TNF receptor Grindelwald couples loss of cell polarity and neoplastic growth. Nature 2015, 522, 482–486. [Google Scholar] [CrossRef]
- Sasaki, M.; Jordan, P.; Joh, T.; Itoh, M.; Jenkins, M.; Pavlick, K.; Minagar, A.; Alexander, S.J. Melatonin reduces TNF-α induced expression of MAdCAM-1 via inhibition of NF-kB. BMC Gastroenterol. 2002, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Wang, G.; Ai, L.; Shi, J.; Zhang, J.; Chen, Y.X. Melatonin suppresses TLR9-triggered proinflammatory cytokine production in macrophages by inhibiting ERK1/2 and AKT activation. Sci. Rep. 2018, 8, 15579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.C.; Chiou, C.H.; Liu, S.C.; Hu, S.L.; Su, C.M.; Tsai, C.H.; Tang, C.H. Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J. Pineal Res. 2019, 66, e12560. [Google Scholar] [CrossRef] [PubMed]
- Saberi, K.; Pasbakhsh, P.; Omidi, A.; Borhani-Haghighi, M.; Nekoonam, S.; Omidi, N.; Ghasemi, S.; Kashani, I.R. Melatonin preconditioning of bone-marrow derived mesenchymal stem cells promotes their engraftment and improves renal regeneration in a rat model of chronic kidney disease. J. Mol. Hist. 2019, 50, 129–140. [Google Scholar] [CrossRef]
- Hill, S.M.; Blask, D.E. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Res. 1988, 48, 6121–6126. [Google Scholar]
- Cos, S.; Fernandez, R.; Guezmes, A.; Sanchez-Barcelo, E.J. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998, 58, 4383–4390. [Google Scholar]
- Papazisis, K.T.; Kouretas, D.; Geromichalos, G.D.; Sivridis, E.; Tsekreli, O.K.; Dimitriadis, K.A.; Kortsaris, A.H. Effects of melatonin on proliferation of cancer cell lines. J. Pineal Res. 1998, 25, 211–218. [Google Scholar] [CrossRef]
- Petranka, J.; Baldwin, W.; Biermann, J.; Jayadev, S.; Barrett, J.C.; Murphy, E. The oncostatic action of melatonin in an ovarian carcinoma cell line. J. Pineal Res. 1999, 26, 129–136. [Google Scholar] [CrossRef]
- Farriol, M.; Venereo, Y.; Orta, X.; Castellanos, J.M.; Segovia-Silvestre, T. In vitro effects of melatonin on cell proliferation in a colon adenocarcinoma line. J. Appl. Toxicol. 2000, 20, 21–24. [Google Scholar] [CrossRef]
- Marelli, M.M.; Limonta, P.; Maggi, R.; Motta, M.; Moretti, R.M. Growth-inhibitory activity of melatonin on human androgen-independent DU 145 prostate cancer cells. Prostate 2000, 45, 238–244. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Cui, P.; Yu, M.; Luo, Z.; Dai, M.; Han, J.; Xiu, R.; Yang, Z. Intracellular signaling pathways involved in cell growth inhibition of human umbilical vein endothelial cells by melatonin. J. Pineal Res. 2008, 44, 107–114. [Google Scholar] [CrossRef]
- Smith, E. Health and disease, as influenced by the daily, seasonal, and other cyclical changes in the human system. In The Edinburgh Medical and Surgical Journal; Walton and Maberly: London, UK, 1861; pp. 1128–1130. [Google Scholar]
- Mills, J.N.; Stanbury, S.W. Persistent 24-hour renal excretory rhythm on a 12-hour cycle of activity. J. Physiol. 1952, 117, 22–37. [Google Scholar] [PubMed]
- Wu, T.; Ni, Y.; Dong, Y.; Xu, J.; Song, X.; Kato, H.; Fu, Z. Regulation of circadian gene expression in the kidney by light and food cues in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R635–R641. [Google Scholar] [CrossRef] [PubMed]
- Hanly, P. Sleep disorders and end-stage renal disease. Curr. Opin. Pulm. Med. 2008, 14, 543–550. [Google Scholar] [CrossRef] [PubMed]
- De Santo, R.M.; Perna, A.; Di Iorio, B.R.; Cirillo, M. Sleep disorders in kidney disease. Minerva Urol. Nefrol. 2010, 62, 111–128. [Google Scholar] [PubMed]
- Pierratos, A.; Hanly, P.J. Sleep disorders over the full range of chronic kidney disease. Blood Purif. 2011, 31, 146–150. [Google Scholar] [CrossRef]
- Ezzat, H.; Mohab, A. Prevalence of sleep disorders among ESRD patients. Ren. Fail. 2015, 37, 1013–1019. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.Y.H.; Hung, C.C.; Chang, Y.H.; Lin, M.Y.; Yang, M.Y.; Liang, S.S.; Liu, W.; Chen, H.C.; Hwang, S.J. Nonapnea sleep disorders in patients younger than 65 years are significantly associated with CKD: A nationwide population-based study. PLoS ONE 2015, 10, e0140401. [Google Scholar] [CrossRef]
- Maung, S.C.; El Sara, A.; Chapman, C.; Cohen, D.; Cukor, D. Sleep disorders and chronic kidney disease. World J. Nephrol. 2016, 5, 224–232. [Google Scholar] [CrossRef]
- Nigam, G.; Camacho, M.; Chang, E.T.; Riaz, M. Exploring sleep disorders in patients with chronic kidney disease. Nat. Sci. Sleep 2018, 10, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edalat-Nejad, M.; Haqhverdi, F.; Hossein-Tabar, T.; Ahmadian, M. Melatonin improves sleep quality in hemodialysis patients. Indian J. Nephrol. 2013, 23, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Lin, K.C.; Wallace, C.G.; Chen, Y.T.; Yang, C.C.; Leu, S.; Chen, Y.C.; Sun, C.K.; Tsai, T.H.; Chen, Y.L.; et al. Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. J. Pineal Res. 2014, 57, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, C.; Zhang, P.; Jiang, H.; Chen, J. Melatonin preconditioning is an effective strategy for mesenchymal stem cell-based therapy for kidney disease. J. Cell. Mol. Med. 2019, 24, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Sozen, M.A.; Armstrong, J.D.; Yang, M.; Kaiser, K.; Dow, J.A.T. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc. Natl. Acad. Sci. USA 1997, 94, 5207–5212. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.T.; Blask, D.E.; Lemus-Wilson, A.M. Melatonin augments the sensitivity of MCF-7 human breast cancer cells to tamoxifen in vitro. J. Clin. Endocrinol. Metab. 1992, 75, 669–670. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Sainz, R.M.; Mayo, J.C.; Lopez-Burillo, S. Melatonin: Reducing the toxicity and increasing the efficacy of drugs. J. Pharm. Pharmacol. 2002, 54, 1299–1321. [Google Scholar] [CrossRef] [Green Version]
- Lissoni, P.; Brivio, F.; Fumagalli, L.; Messina, G.; Vigore, L.; Parolini, D.; Colciago, M.; Rovelli, F. Neuroimmunomodulation in medical oncology: Application of psychoneuroimmunology with subcutaneous low-dose IL-2 and the pineal hormone melatonin in patients with untreatable metastatic solid tumors. Anticancer Res. 2008, 28, 1377–1381. [Google Scholar]
- Ruiz-Rabelo, J.; Vazquez, R.; Arjona, A.; Perea, D.; Montilla, P.; Tunez, I.; Muntane, J.; Padillo, J. Improvement of capecitabine antitumoral activity by melatonin in pancreatic cancer. Pancreas 2011, 40, 410–414. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, S.J.; Kim, B.; Yun, S.M.; Choi, D.Y.; Kim, S.H. Melatonin synergistically enhances cisplatin-induced apoptosis via the dephosphorylation of ERK/p90 ribosomal S6 kinase/heat shock protein 27 in SK-OV-3 cells. J. Pineal Res. 2012, 52, 244–252. [Google Scholar] [CrossRef]
- Margheri, M.; Pacini, N.; Tani, A.; Nosi, D.; Squecco, R.; Dama, A.; Masala, E.; Francini, F.; Zecchi-Orlandini, S.; Formigli, L. Combined effects of melatonin and all-trans retinoic acid and somatostatin on breast cancer cell proliferation and death: Molecular basis for the anticancer effect of these molecules. Eur. J. Pharmacol. 2012, 681, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Uguz, A.C.; Cig, B.; Espino, J.; Bejarano, I.; Naziroglu, M.; Rodriguez, A.B.; Pariente, J.A. Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J. Pineal Res. 2012, 53, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Sohn, E.J.; Shin, E.A.; Lee, D.; Kim, B.; Jung, D.B.; Kim, J.H.; Yun, M.; Lee, H.J.; Park, Y.K.; et al. Melatonin suppresses the expression of 45S preribosomal RNA and upstream binding factor and enhances the antitumor activity of puromycin in MDA-MB-231 breast cancer cells. Evid.-Based Complement. Alternat. Med. 2013, 2013, 879746. [Google Scholar] [CrossRef] [PubMed]
- Kosar, P.A.; Naziroglu, M.; Ovey, I.S.; Cig, B. Synergic effects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in MCF-7 breast cancer cells: Involvement of TRPV1 channels. J. Membr. Biol. 2016, 249, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Woo, S.H.; Oh, S.T.; Hong, S.E.; Choe, T.B.; Ye, S.K.; Kim, E.K.; Seong, M.K.; Kim, H.A.; Noh, W.C.; et al. Melatonin enhances arsenic trioxide-induced cell death via sustained upregulation of Redd1 expression in breast cancer cells. Mol. Cell. Endocrinol. 2016, 422, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hoffman, K.; Gao, C.; Petrulionis, M.; Herr, I.; Schemmer, P. Melatonin promotes sorafenib-induced apoptosis through synergistic activation of JNK/c-jun pathway in human hepatocellular carcinoma. J. Pineal Res. 2017, 62, e12398. [Google Scholar] [CrossRef]
Sample Availability: Melatonin is commercially available. |



| Anterior Tubules | Posterior Tubules | |
|---|---|---|
| BicCΔ/YC33 (n = 50) | 36% (p = 0.0029) | 31% (p = 0.017) |
| BicCΔ/IIF34 (n = 50) | 18% (n.s.) | 7% (n.s.) |
| Anterior Tubules | Posterior Tubules | |||||
|---|---|---|---|---|---|---|
| Proximal | Intermediate | Terminal | Proximal | Intermediate | Terminal | |
| BicCΔ/YC33 | 59% p = 0.0389 | 37% p = 0.0031 | 31% p = 0.0152 | 12% p = 0.3493 | 30% p = 0.0070 | 25% p = 0.0454 |
| BicCΔ/IIF34 | 7% p = 0.9051 | 21% p = 1296 | 16% p = 0.1798 | 2% p = 0.9013 | 9% p = 0.4891 | 7% p = 0.5161 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millet-Boureima, C.; Rozencwaig, R.; Polyak, F.; Gamberi, C. Cyst Reduction by Melatonin in a Novel Drosophila Model of Polycystic Kidney Disease. Molecules 2020, 25, 5477. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25225477
Millet-Boureima C, Rozencwaig R, Polyak F, Gamberi C. Cyst Reduction by Melatonin in a Novel Drosophila Model of Polycystic Kidney Disease. Molecules. 2020; 25(22):5477. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25225477
Chicago/Turabian StyleMillet-Boureima, Cassandra, Roman Rozencwaig, Felix Polyak, and Chiara Gamberi. 2020. "Cyst Reduction by Melatonin in a Novel Drosophila Model of Polycystic Kidney Disease" Molecules 25, no. 22: 5477. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25225477