Synthesis of 3,4-Bis(Butylselanyl)Selenophenes and 4-Alkoxyselenophenes Promoted by Oxone®
Abstract
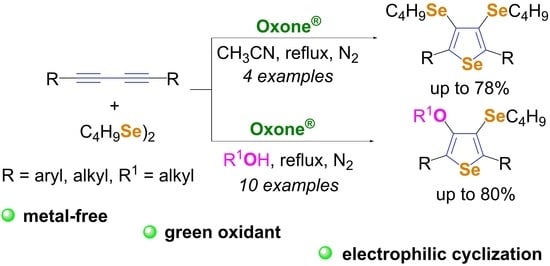
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure for the Synthesis of 3,4-Bis(Butylselanyl)Selenophenes 3
3.2. General Procedure for the Synthesis of 3-(Butylselanyl)-4-Alkoxyselenophenes 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wiles, J.A.; Phadke, A.S.; Bradbury, B.J.; Pucci, M.J.; Thanassi, J.A.; Deshpande, M. Selenophene-Containing Inhibitors of Type IIA Bacterial Topoisomerases. J. Med. Chem. 2011, 54, 3418–3425. [Google Scholar] [CrossRef]
- Wilhelm, E.A.; Jesse, C.R.; Bortolatto, C.F.; Nogueira, C.W.; Savegnago, L. Anticonvulsant and Antioxidant Effects of 3-Alkynyl Selenophene in 21-Day-old Rats on Pilocarpine Model of Seizures. Brain Res. Bull. 2009, 79, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, E.A.; Gai, B.M.; Souza, A.C.G.; Bortolatto, C.F.; Roehrs, J.A.; Nogueira, C.W. Involvement of GABAergic and Glutamatergic Systems in the Anticonvulsant Activity of 3-Alkynyl Selenophene in 21 Day-old Rats. Mol. Cell. Biochem. 2012, 365, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Gai, B.M.; Prigol, M.; Stein, A.L.; Nogueira, C.W. Antidepressant-like Pharmacological Profile of 3-(4-Fluorophenylselenyl)-2,5-diphenylselenophene: Involvement of Serotonergic System. Neuropharmacology 2010, 59, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Gai, B.M.; Stein, A.L.; Roehrs, J.A.; Bilheri, F.N.; Nogueira, C.W.; Zeni, G. Synthesis and Antidepressant-like Activity of Selenophenes Obtained via Iron(III)–PhSeSePh-mediated Cyclization of Z-Selenoenynes. Org. Biomol. Chem. 2012, 10, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Gai, B.M.; Bortolatto, C.F.; Heck, S.O.; Stein, A.L.; Duarte, M.M.M.F.; Zeni, G.; Nogueira, C.W. An Organoselenium Compound Improves Behavioral, Endocrinal and Neurochemical Changes Induced by Corticosterone in Mice. Psychopharmacology 2014, 231, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Gai, B.M.; Bortolatto, C.F.; Brüning, C.A.; Zborowski, V.A.; Stein, A.L.; Zeni, G.; Nogueira, C.W. Depression-Related Behavior and Mechanical Allodynia are Blocked by 3-(4-Fluorophenylselenyl)-2,5-diphenylselenophene in a Mouse Model of Neuropathic Pain Induced by Partial Sciatic Nerve Ligation. Neuropharmacology 2014, 79, 580–589. [Google Scholar] [CrossRef]
- Gai, B.M.; Sanna, M.D.; Stein, A.L.; Zeni, G.; Galeotti, N.; Nogueira, C.W. ERK1/2 Phosphorylation is Involved in the Antidepressant-like Action of 2,5-Diphenyl-3-(4-fluorophenylseleno)-selenophene in Mice. Eur. J. Pharmacol. 2014, 736, 44–54. [Google Scholar] [CrossRef]
- Velasquez, D.; Quines, C.; Pistóia, R.; Zeni, G.; Nogueira, C.W. Selective inhibition of MAO-A Activity Results in an Antidepressant-like Action of 2-Benzoyl 4-iodoselenophene in Mice. Physiol. Behav. 2017, 170, 100–105. [Google Scholar] [CrossRef]
- Schumacher, R.F.; Rosário, A.R.; Souza, A.C.G.; Acker, C.I.; Nogueira, C.W.; Zeni, G. The Potential Antioxidant Activity of 2,3-Dihydroselenophene, a Prototype Drug of 4-Aryl-2,3-dihydroselenophenes. Bioorg. Med. Chem. 2011, 19, 1418–1425. [Google Scholar] [CrossRef] [Green Version]
- Shiah, H.-S.; Lee, W.-S.; Juang, S.-H.; Hong, P.-C.; Lung, C.-C.; Chang, C.-J.; Chou, K.-M.; Chang, J.-Y. Mitochondria-mediated and p53-Associated Apoptosis Induced in Human Cancer Cells by a Novel Selenophene Derivative, D-501036. Biochem. Pharmacol. 2007, 73, 610–619. [Google Scholar] [CrossRef]
- Juang, S.-H.; Lung, C.-C.; Hsu, P.-C.; Hsu, K.-S.; Li, Y.-C.; Hong, P.-C.; Shiah, H.-S.; Kuo, C.-C.; Huang, C.-W.; Wang, Y.-C.; et al. D-501036, a Novel Selenophene-based Triheterocycle Derivative, Exhibits Potent in vitro and in vivo Antitumoral Activity which Involves DNA Damage and Ataxia Telangiectasia–mutated Nuclear Protein Kinase Activation. Mol. Cancer Ther. 2007, 6, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Hu, Z.; Xiao, Y.; Yang, T.; Dong, C.; Huang, J.; Zhou, H.-B. Rational design and optimization of selenophenes with basic side chains as novel potent selective estrogen receptor modulators (SERMs) for breast cancer therapy. Med. Chem. Commun. 2017, 8, 1485–1497. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Hu, Z.; Li, C.; Tang, C.; Carlson, K.E.; Luo, J.; Dong, C.; Katzenellenbogen, J.A.; Huang, J.; et al. Selenophenes: Introducing a New Element into the Core of Non-Steroidal Estrogen Receptor Ligands. ChemMedChem 2017, 12, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liang, P.; Si, W.; Zhao, B.; Shao, J.; Huang, W.; Zhang, Y.; Zhang, Q.; Dong, X. A Selenophene Substituted Diketopyrrolopyrrole Nanotheranostic Agent for Highly Efficient Photoacoustic/Infrared-thermal Imaging-guided Phototherapy. Org. Chem. Front. 2018, 5, 98–105. [Google Scholar] [CrossRef]
- Wilhelm, E.A.; Jesse, C.R.; Roman, S.S.; Nogueira, C.W.; Savegnago, L. Hepatoprotective Effect of 3-Alkynyl Selenophene on Acute Liver Injury Induced by D-Galactosamine and Lipopolysaccharide. Exp. Mol. Pathol. 2009, 87, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, E.A.; Jesse, C.R.; Prigol, M.; Alves, D.; Schumacher, R.F.; Nogueira, C.W. 3-Alkynyl Selenophene Protects Against Carbon-tetrachloride-induced and 2-Nitropropane-induced Hepatic Damage in Rats. Cell Biol. Toxicol. 2010, 26, 569–577. [Google Scholar] [CrossRef]
- Wilhelm, E.A.; Jesse, C.R.; Bortolatto, C.F.; Nogueira, C.W.; Savegnago, L. Antinociceptive and Anti-allodynic Effects of 3-Alkynyl Selenophene on Different Models of Nociception in Mice. Pharmacol. Biochem. Behav. 2009, 93, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.B.A.; Kirsch, G.; Peagle, E. Biologically Active Selenophenes and Benzo[b]selenophenes. Curr. Org. Synth. 2017, 14, 1091–1101. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Xu, C.; Okamoto, T. Ladder π-Conjugated Materials with Main Group Elements. Pure Appl. Chem. 2006, 78, 721–730. [Google Scholar] [CrossRef]
- Mohanakrishnan, A.K.; Kumar, N.S.; Amaladass, P. Synthesis and Characterization of 9,9-Dialkylfluorene Capped Benzo[c]thiophene/Benzo[c]selenophene Analogs as Potential OLEDs. Tetrahedron Lett. 2008, 49, 4792–4795. [Google Scholar] [CrossRef]
- Cho, W.; Sarada, G.; Lee, H.; Song, M.; Gal, Y.-S.; Lee, Y.; Jin, S.-H. Highly Efficient, Conventional and Flexible Deep-red Phosphorescent OLEDs Using Ambipolar Thiophene/Selenophene-phenylquinoline Ligand-based Ir(III) Complexes. Dye. Pigment. 2017, 136, 390–397. [Google Scholar] [CrossRef]
- Petrenko, A.; Leitonas, K.; Volyniuk, D.; Baryshnikov, G.V.; Belyakov, S.; Minaev, B.F.; Ågren, H.; Durgaryan, H.; Gražulevičius, J.V.; Arsenyan, P. Benzoselenophenylpyridine Platinum Complexes: Green versus Red Phosphorescence Towards Hybrid OLEDs. Dalton Trans. 2020, 49, 3393–3397. [Google Scholar] [CrossRef] [PubMed]
- Takimiya, K.; Kunugi, Y.; Konda, Y.; Ebata, H.; Toyoshima, Y.; Otsubo, T. 2,7-Diphenyl[1]benzoselenopheno[3,2-b][1]benzoselenophene as a Stable Organic Semiconductor for a High-Performance Field-Effect Transistor. J. Am. Chem. Soc. 2006, 128, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Takimiya, K. Facile Synthesis of Highly π-Extended Heteroarenes, Dinaphtho[2,3-b:2‘,3‘-f]chalcogenopheno[3,2-b]chalcogenophenes, and Their Application to Field-Effect Transistors. J. Am. Chem. Soc. 2007, 129, 2224–2225. [Google Scholar] [CrossRef]
- Shan, Z.; Shi, J.; Xu, W.; Li, C.; Wang, H. Organic Field-effect Transistors Based on Biselenophene Derivatives as Active Layers. Dye. Pigment. 2019, 171, 107675. [Google Scholar] [CrossRef]
- Gao, D.; Hollinger, J.; Seferos, D.S. Selenophene-Thiophene Block Copolymer Solar Cells with Thermostable Nanostructures. ACS Nano 2012, 6, 7114–7121. [Google Scholar] [CrossRef]
- Ashraf, R.S.; Meager, I.; Nikolka, M.; Kirkus, M.; Planells, M.; Schroeder, B.C.; Holliday, S.; Hurhangee, M.; Nielsen, C.B.; Sirringhaus, H.; et al. Chalcogenophene Comonomer Comparison in Small Band Gap Diketopyrrolopyrrole-based Conjugated Polymers for High-performing Field-effect Transistors and Organic Solar Cells. J. Am. Chem. Soc. 2015, 137, 1314–1321. [Google Scholar] [CrossRef]
- Meng, D.; Sun, D.; Zhong, C.; Liu, T.; Fan, B.; Huo, L.; Li, Y.; Jiang, W.; Choi, H.; Kim, T.; et al. High-Performance Solution-Processed Non-Fullerene Organic Solar Cells Based on Selenophene-Containing Perylene Bisimide Acceptor. J. Am. Chem. Soc. 2016, 138, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.-Y.; Tseng, C.-C.; Lin, F.-Y.; Chen, Y.; Yan, H.; Cheng, Y.-J. Selenophene-Incorporated Quaterchalcogenophene-Based Donor-Acceptor Copolymers to Achieve Efficient Solar Cells with Jsc Exceeding 20 mA/cm2. Chem. Mater. 2017, 29, 10045–10052. [Google Scholar] [CrossRef]
- Li, C.; Xia, T.; Song, J.; Fu, H.; Ryu, H.S.; Weng, K.; Ye, L.; Woo, H.Y.; Sun, Y. Asymmetric Selenophene-based Non-fullerene Acceptors for High-performance Organic Solar Cells. J. Mater. Chem. A 2019, 7, 1435–1441. [Google Scholar] [CrossRef]
- Kong, H.; Chung, D.S.; Kang, I.-N.; Park, J.-H.; Park, M.-J.; Jung, I.H.; Park, C.E.; Shim, H.-K. New Selenophene-based Semiconducting Copolymers for High Performance Organic Thin-film Transistors. J. Mater. Chem. 2009, 19, 3490–3499. [Google Scholar] [CrossRef] [Green Version]
- Kong, H.; Jung, Y.K.; Cho, N.S.; Kang, I.-N.; Park, J.-H.; Cho, S.; Shim, H.-K. New Semiconducting Polymers Containing 3,6-Dimethyl(thieno[3,2-b]thiophene or Selenopheno[3,2-b]selenophene) for Organic Thin-film Transistors. Chem. Mater. 2009, 21, 2650–2660. [Google Scholar] [CrossRef]
- Fei, Z.; Han, Y.; Gann, E.; Hodsden, T.; Chesman, A.S.R.; McNeill, C.R.; Anthopoulos, T.D.; Heeney, M. Alkylated Selenophene-Based Ladder-Type Monomers via a Facile Route for High-Performance Thin-Film Transistor Applications. J. Am. Chem. Soc. 2017, 139, 8552–8561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, O.S.R.; Favero, A.; Nogueira, C.W.; Menezes, P.H.; Zeni, G. Palladium-catalyzed Cross-coupling of 2-Haloselenophene with Terminal Alkynes in the Absence of Additive. Tetrahedron Lett. 2006, 47, 2179–2182. [Google Scholar] [CrossRef]
- Prediger, P.; Moro, A.V.; Nogueira, C.W.; Savegnago, L.; Rocha, J.B.T.; Zeni, G. Palladium-Catalyzed Suzuki Cross-Coupling of 2-Haloselenophenes: Synthesis of 2-Arylselenophenes, 2,5-Diarylselenophenes, and 2-Arylselenophenyl Ketones. J. Org. Chem. 2006, 71, 3786–3792. [Google Scholar] [CrossRef]
- Schumacher, R.F.; Alves, D.; Brandão, R.; Nogueira, C.W.; Zeni, G. 3-Iodoselenophene Derivatives: A Versatile Substrate for Negishi Cross-coupling Reaction. Tetrahedron Lett. 2008, 49, 538–542. [Google Scholar] [CrossRef]
- Alves, D.; Reis, J.S.; Luchese, C.; Nogueira, C.W.; Zeni, G. Synthesis of 3-Alkynylselenophene Derivatives by a Copper-Free Sonogashira Cross-Coupling Reaction. Eur. J. Org. Chem. 2008, 2008, 377–382. [Google Scholar] [CrossRef]
- Tùng, D.T.; Villinger, A.; Langer, P. Efficient Synthesis of Substituted Selenophenes Based on the First Palladium(0)-Catalyzed Cross-Coupling Reactions of Tetrabromoselenophene. Adv. Synth. Catal. 2008, 350, 2109–2117. [Google Scholar] [CrossRef]
- Skhiri, A.; Salem, R.B.; Soulé, J.-F.; Doucet, H. Reactivity of Bromoselenophenes in Palladium-catalyzed Direct Arylations. Beilstein J. Org. Chem. 2017, 13, 2862–2868. [Google Scholar] [CrossRef] [Green Version]
- Skhiri, A.; Salem, R.B.; Soul, J.-F.; Doucet, H. Access to (Hetero)arylated Selenophenes via Palladium catalysed Stille, Negishi or Suzuki Couplings or C-H Bond Functionalization Reaction. ChemCatChem 2017, 9, 2895–2913. [Google Scholar] [CrossRef] [Green Version]
- Barros, O.S.R.; Nogueira, C.W.; Stangherlin, E.C.; Menezes, P.H.; Zeni, G. Copper-Promoted Carbon-Nitrogen Bond Formation with 2-Iodo-selenophene and Amides. J. Org. Chem. 2006, 71, 1552–1557. [Google Scholar] [CrossRef]
- Prediger, P.; Brandão, R.; Nogueira, C.W.; Zeni, G. Palladium-Catalyzed Carbonylation of 2-Haloselenophenes: Synthesis of Selenophene-2-carboxamides, Selenophene-2,5-dicarboxamides and N,N′-Bridged Selenophene-2-carboxamides. Eur. J. Org. Chem. 2007, 32, 5422–5428. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Li, L.-C.; Yang, C.; Li, Y.-R. Theoretical Investigation on the Mechanism of the CuI-Catalyzed Carbon−Nitrogen Coupling Reaction of 2-Iodo-selenophene with Benzamide. J. Phys. Chem. A 2008, 112, 435–440. [Google Scholar] [CrossRef]
- Zeni, G. Carbon–Sulfur Bond Formation from 2-Halochalcogenophenes via Copper Catalyzed Thiol Cross-coupling. Tetrahedron Lett. 2005, 46, 2647–2651. [Google Scholar] [CrossRef]
- Tamba, S.; Fujii, R.; Mori, A.; Hara, K.; Koumura, N. Synthesis and Properties of Seleno-analog MK-organic Dye for Photovoltaic Cells Prepared by C-H Functionalization Reactions of Selenophene Derivatives. Chem. Lett. 2011, 40, 922–924. [Google Scholar] [CrossRef] [Green Version]
- Rampon, D.S.; Wessjohann, L.A.; Schneider, P.H. Palladium-Catalyzed Direct Arylation of Selenophene. J. Org. Chem. 2014, 79, 5987–5992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skhiri, A.; Salem, R.B.; Soule, J.-F.; Doucet, H. Unprecedented Access to β-Arylated Selenophenes through Palladium-Catalysed Direct Arylation. Chem. Eur. J. 2017, 23, 2788–2791. [Google Scholar] [CrossRef]
- Shi, X.; Mao, S.; Roisnel, T.; Doucet, H.; Soule, J.-F. Palladium-catalyzed Successive C–H Bond Arylations and Annulations Toward the π-Extension of Selenophene-containing Aromatic Skeletons. Org. Chem. Front. 2019, 6, 2398–2403. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Sahoo, S.K.; Huang, C.-L.; Chan, T.-H.; Cheng, Y.-J. Pd(II)-Catalyzed Direct Dehydrogenative Mono- and Diolefination of Selenophenes. Org. Lett. 2020, 22, 2318–2322. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, P.S.; Peglow, T.J.; Penteado, F.; Bagnoli, L.; Perin, G.; Lenardão, E.J. Recent Advances in the Synthesis of Selenophenes and Their Derivatives. Molecules 2020, 25, 5907. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Luchese, C.; Nogueira, C.W.; Zeni, G. Electrophilic Cyclization of (Z)-Selenoenynes: Synthesis and Reactivity of 3-Iodoselenophenes. J. Org. Chem. 2007, 72, 6726–6734. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.K.; Barcellos, A.M.; Neto, J.S.S.; Alves, D.; Lenardão, E.J.; Rosati, O.; Santi, C.; Perin, G. Dichalcogenides/Oxone®-Mediated Cyclization of (Z)-Chalcogenoenynes under Ultrasound Irradiation. ChemistrySelect 2020, 5, 9813–9819. [Google Scholar] [CrossRef]
- Pistoia, R.P.; Roehrs, J.A.; Back, D.F.; Zeni, G. Iodine-mediated Regioselective 5-Endo-dig Electrophilic Cyclization Reaction of Selenoenynes: Synthesis of Selenophene Derivatives. Org. Chem. Front. 2017, 4, 277–282. [Google Scholar] [CrossRef]
- Bilheri, F.N.; Stein, A.L.; Zeni, G. Synthesis of Chalcogenophenes via Cyclization of 1,3-Diynes Promoted by Iron(III) Chloride and Dialkyl Dichalcogenides. Adv. Synth. Catal. 2015, 357, 1221–1228. [Google Scholar] [CrossRef]
- Siroos, Z.; Kassanabadi, A.; Mosslemin, M.A. A Synthesis of Novel Selenophenes from Activated Acetylenes, Ethyl 2-Cyano-3-arylacrylate and Potassium Selenocyanate. Org. Prep. Proced. Int. 2021. [Google Scholar] [CrossRef]
- Perin, G.; Soares, L.K.; Hellwig, P.S.; Silva, M.S.; Neto, J.S.S.; Roehrs, J.A.; Barcellos, T.; Lenardão, E.J. Synthesis of 2,3-Bis-organochalcogenyl-benzo[b]chalcogenophenes Promoted by Oxone®. New J. Chem. 2019, 43, 6323–6331. [Google Scholar] [CrossRef]
- Perin, G.; Nobre, P.C.; Mailahn, D.H.; Silva, M.S.; Barcellos, T.; Jacob, R.G.; Lenardão, E.J.; Santi, C.; Roehrs, J.A. Synthesis of 4-Organoselanyl-1H-pyrazoles: Oxone®-Mediated Electrophilic Cyclization of α,β-Alkynyl Hydrazones by Using Diorganyl Diselenides. Synthesis 2019, 51, 2293–2304. [Google Scholar] [CrossRef]
- Goulart, H.A.; Neto, J.S.S.; Barcellos, A.M.; Barcellos, T.; Silva, M.S.; Alves, D.; Jacob, R.G.; Lenardão, E.J.; Perin, G. Synthesis of 5H-Selenopheno[3,2-c]isochromen-5-ones Promoted by Dialkyl Diselenides and Oxone®. Adv. Synth. Catal. 2019, 361, 3403–3411. [Google Scholar] [CrossRef]
- Araujo, D.R.; Goulart, H.A.; Barcellos, A.M.; Cargnelutti, R.; Lenardão, E.J.; Perin, G. Oxone-Promoted Synthesis of 4-(Chalcogenyl)isoquinoline-N-oxides from Alkynylbenzaldoximes and Diorganyl Dichalcogenides. J. Org. Chem. 2021, 86, 1721–1729. [Google Scholar] [CrossRef]
- Hellwig, P.S.; Guedes, J.S.; Barcellos, A.M.; Jacob, R.G.; Silveira, C.C.; Lenardão, E.J.; Perin, G. Synthesis of Benzo[b]chalcogenophenes Fused to Selenophenes via Intramolecular Electrophilic Cyclization of 1,3-Diynes. Org. Biomol. Chem. 2021, 19, 596–604. [Google Scholar] [CrossRef] [PubMed]




| # | 2a (mmol) | Oxone® (mmol) | Solvent | Temp. | Time (h) | Yield 3a (%) b | Yield 4a (%) b |
|---|---|---|---|---|---|---|---|
| 1 | 0.50 | 0.50 | CH3CN | 80 °C | 72 | 58 | - |
| 2 c | 0.50 | 0.50 | CH3CN | - | 2 | 15 | - |
| 3 | 0.50 | 0.50 | DMF | 110 °C | 72 | NR d | - |
| 4 | 0.50 | 0.50 | PEG-400 | 90 °C | 72 | NR d | - |
| 5 | 0.50 | 0.50 | glycerol | 90 °C | 72 | NR d | - |
| 6 | 0.50 | 0.50 | EtOH | reflux | 24 | 15 | 43 |
| 7 | 0.50 | 0.75 | CH3CN | 80 °C | 48 | 78 | - |
| 8 | 0.50 | 1.0 | CH3CN | 80 °C | 48 | 75 | - |
| 9 | 0.50 | 0.25 | CH3CN | 80 °C | 72 | 38 | - |
| 10 | 0.50 | 0.38 | CH3CN | 80 °C | 72 | 50 | - |
| 11 | 0.38 | 0.50 | EtOH | reflux | 24 | 12 | 48 |
| 12 | 0.25 | 0.50 | EtOH | reflux | 36 | 5 | 35 |
| 13 | 0.38 | 0.25 | EtOH | reflux | 48 | 8 | 39 |
| 14 | 0.38 | 0.38 | EtOH | reflux | 36 | 7 | 42 |
| 15 | 0.38 | 0.75 | EtOH | reflux | 24 | 3 | 70 |
| 16 | 0.38 | 1.0 | EtOH | reflux | 24 | 5 | 65 |
| 17 e | 0.38 | 0.75 | EtOH | reflux | 48 | 8 | 15 |
 |
 |
 |
 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellwig, P.S.; Guedes, J.S.; Barcellos, A.M.; Perin, G.; Lenardão, E.J. Synthesis of 3,4-Bis(Butylselanyl)Selenophenes and 4-Alkoxyselenophenes Promoted by Oxone®. Molecules 2021, 26, 2378. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26082378
Hellwig PS, Guedes JS, Barcellos AM, Perin G, Lenardão EJ. Synthesis of 3,4-Bis(Butylselanyl)Selenophenes and 4-Alkoxyselenophenes Promoted by Oxone®. Molecules. 2021; 26(8):2378. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26082378
Chicago/Turabian StyleHellwig, Paola S., Jonatan S. Guedes, Angelita M. Barcellos, Gelson Perin, and Eder J. Lenardão. 2021. "Synthesis of 3,4-Bis(Butylselanyl)Selenophenes and 4-Alkoxyselenophenes Promoted by Oxone®" Molecules 26, no. 8: 2378. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26082378