Fluorescent Phthalocyanine-Encapsulated Bovine Serum Albumin Nanoparticles: Their Deployment as Therapeutic Agents in the NIR Region
Abstract
:1. Introduction
2. Results
2.1. Optical Characterization of PhthaloNH2
2.2. PhthaloNH2 Loaded BSA NPs
2.2.1. Spectroscopic and Morphologic Characterization
2.2.2. BSA NPs Yield and phthaloNH2 Loading Efficiency
2.2.3. Molecular Docking Simulation
2.3. Free vs. Encapsulated PhthaloNH2 withdin BSA NPs
2.3.1. Temperature-Dependent Spectroscopic Characterization
2.3.2. MTT Assay
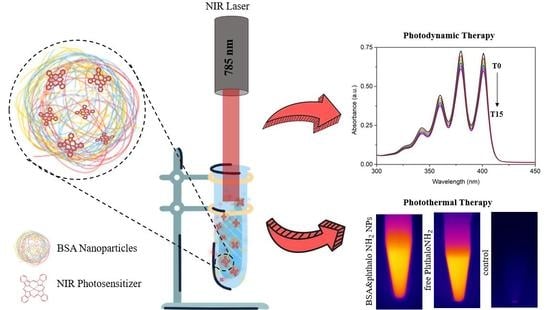
2.3.3. Photodynamic Effects
2.3.4. Photothermal Effects
2.3.5. Photothermal and Size Stability
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PhthaloNH2
4.3. Design of BSA&phthaloNH2 NPs
4.4. Particle Yield and Dye Loading Efficiency
4.5. Molecular Docking
4.6. Cell Viability
4.7. Photodynamic and Photothermal Effects
4.8. Characterization Techniques
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The Future of Cancer Diagnosis and Therapy. CA Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, L.; Wang, J.; He, Y.; Xin, J.; Wang, S.; Xu, H.; Zhang, Z. Gold Nanoparticle Mediated Phototherapy for Cancer. J. Nanomater. 2016, 2016, 5497136. [Google Scholar] [CrossRef] [Green Version]
- Campu, A.; Focsan, M.; Lerouge, F.; Borlan, R.; Tie, L.; Rugina, D.; Astilean, S. ICG-Loaded Gold Nano-Bipyramids with NIR Activatable Dual PTT-PDT Therapeutic Potential in Melanoma Cells. Colloids Surf. B Biointerfaces 2020, 194, 111213. [Google Scholar] [CrossRef]
- Baydoun, M.; Moralès, O.; Frochot, C.; Ludovic, C.; Leroux, B.; Thecua, E.; Ziane, L.; Grabarz, A.; Kumar, A.; de Schutter, C.; et al. Photodynamic Therapy Using a New Folate Receptor-Targeted Photosensitizer on Peritoneal Ovarian Cancer Cells Induces the Release of Extracellular Vesicles with Immunoactivating Properties. J. Clin. Med. 2020, 9, 1185. [Google Scholar] [CrossRef]
- Champeau, M.; Vignoud, S.; Mortier, L.; Mordon, S. Photodynamic Therapy for Skin Cancer: How to Enhance Drug Penetration? J. Photochem. Photobiol. B Biol. 2019, 197, 111544. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y. Gold Nanostructures for Cancer Imaging and Therapy. In Advances in Nanotheranostics I; Dai, Z., Ed.; Springer Series in Biomaterials Science and Engineering; Springer: Berlin, Germany, 2016; Volume 6, pp. 53–101. ISBN 978-3-662-48542-2. [Google Scholar]
- Eskiizmir, G.; Baskın, Y.; Yapıcı, K. Graphene-based nanomaterials in cancer treatment and diagnosis. In Fullerens, Graphenes and Nanotubes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 331–374. ISBN 978-0-12-813691-1. [Google Scholar]
- Sheng, Z.; Cai, L. Organic Dye-Loaded Nanoparticles for Imaging-Guided Cancer Therapy. In Advances in Nanotheranostics I; Dai, Z., Ed.; Springer Series in Biomaterials Science and Engineering; Springer: Berlin, Germany, 2016; Volume 6, ISBN 978-3-662-48542-2. [Google Scholar]
- Rajkumar, S.; Prabaharan, M. Multi-Functional FITC-Silica@gold Nanoparticles Conjugated with Guar Gum Succinate, Folic Acid and Doxorubicin for CT/Fluorescence Dual Imaging and Combined Chemo/PTT of Cancer. Colloids Surf. B Biointerfaces 2020, 186, 110701. [Google Scholar] [CrossRef]
- Feng, J.; Chang, D.; Wang, Z.; Shen, B.; Yang, J.; Jiang, Y.; Ju, S.; He, N. A FITC-Doped Silica Coated Gold Nanocomposite for Both in Vivo X-Ray CT and Fluorescence Dual Modal Imaging. RSC Adv. 2014, 4, 51950–51959. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oniszczuk, A.; Wojtunik-Kulesza, K.A.; Oniszczuk, T.; Kasprzak, K. The Potential of Photodynamic Therapy (PDT)—Experimental Investigations and Clinical Use. Biomed. Pharmacother. 2016, 83, 912–929. [Google Scholar] [CrossRef]
- Van Leeuwen, F.W.B.; Hardwick, J.C.H.; van Erkel, A.R. Luminescence-Based Imaging Approaches in the Field of Interventional Molecular Imaging. Radiology 2015, 276, 12–29. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Hao, X.; Zhang, X.; Zhou, M.; Lee, C.-S.; Chen, X. Near-Infrared Fluorescence Imaging Using Organic Dye Nanoparticles. Biomaterials 2014, 35, 3356–3364. [Google Scholar] [CrossRef] [Green Version]
- Ptaszek, M. Rational Design of Fluorophores for In Vivo Applications. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 113, pp. 59–108. ISBN 978-0-12-386932-6. [Google Scholar]
- Nalwa, H.S. (Ed.) Supramolecular Photosensitive and Electroactive Materials; Academic Press: San Diego, CA, USA, 2001; ISBN 978-0-12-513904-5. [Google Scholar]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as Medicinal Photosensitizers: Developments in the Last Five Years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Wong, R.C.H.; Lo, P.-C.; Ng, D.K.P. Stimuli Responsive Phthalocyanine-Based Fluorescent Probes and Photosensitizers. Coord. Chem. Rev. 2019, 379, 30–46. [Google Scholar] [CrossRef]
- Pereira, G.F.M.; Tasso, T.T. From Cuvette to Cells: How the Central Metal Ion Modulates the Properties of Phthalocyanines and Porphyrazines as Photosensitizers. Inorg. Chim. Acta 2021, 519, 120271. [Google Scholar] [CrossRef]
- Teixeira Tasso, T.; Yamasaki, Y.; Furuyama, T.; Kobayashi, N. An Exemplary Relationship between the Extent of Cofacial Aggregation and Fluorescence Quantum Yield as Exhibited by Quaternized Amphiphilic Phthalocyanines. Dalton Trans. 2014, 43, 5886–5892. [Google Scholar] [CrossRef]
- Sandra, F.; Khaliq, N.U.; Sunna, A.; Care, A. Developing Protein-Based Nanoparticles as Versatile Delivery Systems for Cancer Therapy and Imaging. Nanomaterials 2019, 9, 1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-Based Nanoparticles: From Preparation to Encapsulation of Active Molecules. Int. J. Pharm. 2017, 522, 172–197. [Google Scholar] [CrossRef]
- Pansare, V.J.; Hejazi, S.; Faenza, W.J.; Prud’homme, R.K. Review of Long-Wavelength Optical and NIR Imaging Materials: Contrast Agents, Fluorophores, and Multifunctional Nano Carriers. Chem. Mater. 2012, 24, 812–827. [Google Scholar] [CrossRef] [Green Version]
- Madamsetty, V.S.; Mukherjee, A.; Mukherjee, S. Recent Trends of the Bio-Inspired Nanoparticles in Cancer Theranostics. Front. Pharmacol. 2019, 10, 1264. [Google Scholar] [CrossRef]
- Gou, Y.; Miao, D.; Zhou, M.; Wang, L.; Zhou, H.; Su, G. Bio-Inspired Protein-Based Nanoformulations for Cancer Theranostics. Front. Pharmacol. 2018, 9, 421. [Google Scholar] [CrossRef]
- Borlan, R.; Tatar, A.-S.; Soritau, O.; Maniu, D.; Marc, G.; Florea, A.; Focsan, M.; Astilean, S. Design of Fluorophore-Loaded Human Serum Albumin Nanoparticles for Specific Targeting of NIH:OVCAR3 Ovarian Cancer Cells. Nanotechnology 2020, 31, 315102. [Google Scholar] [CrossRef]
- Cong, F.-D.; Ning, B.; Du, X.-G.; Ma, C.-Y.; Yu, H.-F.; Chen, B. Facile Synthesis, Characterization and Property Comparisons of Tetraaminometallophthylocyanines with and without Intramolecular Hydrogen Bonds. Dye. Pigment. 2005, 66, 149–154. [Google Scholar] [CrossRef]
- Alzeer, J.; Roth, P.J.C.; Luedtke, N.W. An Efficient Two-Step Synthesis of Metal-Free Phthalocyanines Using a Zn(II) Template. Chem. Commun. 2009, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borlan, R.; Focsan, M.; Perde-Schrepler, M.; Soritau, O.; Campu, A.; Gaina, L.; Pall, E.; Pop, B.; Baldasici, O.; Gherman, C.; et al. Antibody Functionalized Theranostic Protein Nanoparticles for Synergistic Deep Red Fluorescence Imaging and Multimodal Therapy of Ovarian Cancer. Biomater. Sci. 2021. [Google Scholar] [CrossRef]
- Diac, A.; Focsan, M.; Socaci, C.; Gabudean, A.-M.; Farcau, C.; Maniu, D.; Vasile, E.; Terec, A.; Veca, L.M.; Astilean, S. Covalent Conjugation of Carbon Dots with Rhodamine B and Assessment of Their Photophysical Properties. RSC Adv. 2015, 5, 77662–77669. [Google Scholar] [CrossRef]
- Hong, N.Y.; Kim, H.R.; Lee, H.M.; Sohn, D.K.; Kim, K.G. Fluorescent Property of Indocyanine Green (ICG) Rubber Ring Using LED and Laser Light Sources. Biomed. Opt. Express 2016, 7, 1637. [Google Scholar] [CrossRef] [Green Version]
- Sibata, M.N.; Tedesco, A.C.; Marchetti, J.M. Photophysicals and Photochemicals Studies of Zinc(II) Phthalocyanine in Long Time Circulation Micelles for Photodynamic Therapy Use. Eur. J. Pharm. Sci. 2004, 23, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Tayyab, S.; Sam, S.E.; Kabir, M.Z.; Ridzwan, N.F.W.; Mohamad, S.B. Molecular Interaction Study of an Anticancer Drug, Ponatinib with Human Serum Albumin Using Spectroscopic and Molecular Docking Methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 199–206. [Google Scholar] [CrossRef]
- Parsekar, S.U.; Velankanni, P.; Sridhar, S.; Haldar, P.; Mate, N.A.; Banerjee, A.; Antharjanam, P.S.; Koley, A.P.; Kumar, M. Protein Binding Studies with Human Serum Albumin, Molecular Docking and in Vitro Cytotoxicity Studies Using HeLa Cervical Carcinoma Cells of Cu(II)/Zn(II) Complexes Containing a Carbohydrazone Ligand. Dalton Trans. 2020, 49, 2947–2965. [Google Scholar] [CrossRef]
- Sakurama, K.; Kawai, A.; Tuan Giam Chuang, V.; Kanamori, Y.; Osa, M.; Taguchi, K.; Seo, H.; Maruyama, T.; Imoto, S.; Yamasaki, K.; et al. Analysis of the Binding of Aripiprazole to Human Serum Albumin: The Importance of a Chloro-Group in the Chemical Structure. ACS Omega 2018, 3, 13790–13797. [Google Scholar] [CrossRef]
- Wang, Z.; Ho, J.X.; Ruble, J.R.; Rose, J.; Rüker, F.; Ellenburg, M.; Murphy, R.; Click, J.; Soistman, E.; Wilkerson, L.; et al. Structural Studies of Several Clinically Important Oncology Drugs in Complex with Human Serum Albumin. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 5356–5374. [Google Scholar] [CrossRef] [PubMed]
- Arfin, S.; Siddiqui, G.A.; Naeem, A.; Moin, S. Inhibition of Advanced Glycation End Products by Isoferulic Acid and Its Free Radical Scavenging Capacity: An in Vitro and Molecular Docking Study. Int. J. Biol. Macromol. 2018, 118, 1479–1487. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Rajendran, T.; Murugan, K.S.; Ganesan, M.; Sivasubramanian, V.K.; Rajagopal, S. Synthesis, Photophysics and the Binding Studies of Rhenium(I) Diimine Surfactant Complexes with Serum Albumins: A Spectroscopic and Docking Study Approach. J. Lumin. 2019, 205, 51–60. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Habash, R.W.Y.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal Therapy, Part 1: An Introduction to Thermal Therapy. Crit. Rev. Biomed. Eng. 2006, 34, 459–489. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Huang, J.; Wang, H. Probing the Interaction between Human Serum Albumin and 9-Hydroxyphenanthrene: A Spectroscopic and Molecular Docking Study. ACS Omega 2020, 5, 16833–16840. [Google Scholar] [CrossRef] [PubMed]
- Nnyigide, O.S.; Lee, S.-G.; Hyun, K. In Silico Characterization of the Binding Modes of Surfactants with Bovine Serum Albumin. Sci. Rep. 2019, 9, 10643. [Google Scholar] [CrossRef] [Green Version]
- Vieira, T.F.; Sousa, S.F. Comparing AutoDock and Vina in Ligand/Decoy Discrimination for Virtual Screening. Appl. Sci. 2019, 9, 4538. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal Structure of Human Serum Albumin at 2.5 Å Resolution. Protein Eng. Des. Sel. 1999, 12, 439–446. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoica, C.I.; Marc, G.; Pîrnău, A.; Vlase, L.; Araniciu, C.; Oniga, S.; Palage, M.; Oniga, O. Thiazolyl-Oxadiazole Derivatives Targeting Lanosterol 14α-Demethylase as Potential Antifungal Agents: Design, Synthesis and Molecular Docking Studies. Farmacia 2016, 64, 390–397. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2010, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adarsh, N.; Avirah, R.R.; Ramaiah, D. Tuning Photosensitized Singlet Oxygen Generation Efficiency of Novel Aza-BODIPY Dyes. Org. Lett. 2010, 12, 5720–5723. [Google Scholar] [CrossRef] [PubMed]











| Sudlow’s Sites | AutoDock | Autodock Vina | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔG (kcal/mol) | Ki (nM) | Mean ΔG (kcal/mol) | ΔG SD | NC | Other Clusters | ΔG (kcal/mol) | Mean ΔG (kcal/mol) | ΔG SD | ||
| NCoC | NoCl | |||||||||
| Site I | −10.79 | 12.23 | −10.78 | 0.01 | 500 | 0 | 0 | −10.10 | −9.50 | 0.44 |
| Site II | −8.80 | 352.01 | −8.73 | 0.05 | 294 | 206 | 2 | −9.00 | −7.95 | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borlan, R.; Stoia, D.; Gaina, L.; Campu, A.; Marc, G.; Perde-Schrepler, M.; Silion, M.; Maniu, D.; Focsan, M.; Astilean, S. Fluorescent Phthalocyanine-Encapsulated Bovine Serum Albumin Nanoparticles: Their Deployment as Therapeutic Agents in the NIR Region. Molecules 2021, 26, 4679. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26154679
Borlan R, Stoia D, Gaina L, Campu A, Marc G, Perde-Schrepler M, Silion M, Maniu D, Focsan M, Astilean S. Fluorescent Phthalocyanine-Encapsulated Bovine Serum Albumin Nanoparticles: Their Deployment as Therapeutic Agents in the NIR Region. Molecules. 2021; 26(15):4679. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26154679
Chicago/Turabian StyleBorlan, Raluca, Daria Stoia, Luiza Gaina, Andreea Campu, Gabriel Marc, Maria Perde-Schrepler, Mihaela Silion, Dana Maniu, Monica Focsan, and Simion Astilean. 2021. "Fluorescent Phthalocyanine-Encapsulated Bovine Serum Albumin Nanoparticles: Their Deployment as Therapeutic Agents in the NIR Region" Molecules 26, no. 15: 4679. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26154679