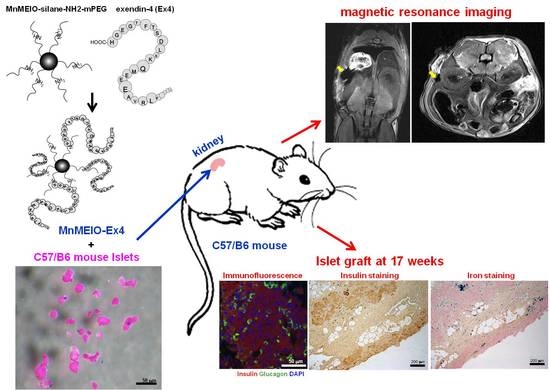
Exendin-4-Conjugated Manganese Magnetism-Engineered Iron Oxide Nanoparticles as a Potential Magnetic Resonance Imaging Contrast Agent for Tracking Transplanted β-Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MnMEIO and MnMEIO-Ex4 NPs
2.3. Characterization of MnMEIO and MnMEIO-Ex4 NPs
2.4. Culture of MIN6 Cells
2.5. In Vitro Cytotoxicity Assay of MnMEIO and MnMEIO-Ex4 NPs
2.6. Insulin Secretion of MIN6 Cells
2.7. Cellular Uptake of MnMEIO and MnMEIO-Ex4 NPs
2.8. Transmission Electron Microscope (TEM) Measurements
2.9. In Vitro MR Scanning
2.10. Animals
2.11. Islet Isolation and Labeling
2.12. Islet Transplantation
2.13. In Vivo MR Scanning
2.14. Islet Graft Removal and Histological Studies
2.15. Statistical Analysis
3. Results
3.1. General Characterization of MnMEIO and MnMEIO-Ex4 NPs
3.2. Effects of MnMEIO and MnMEIO-Ex4 NPs on MIN6 Cell Viability and Insulin Secretion
3.3. Cellular Uptake of MnMEIO and MnMEIO-Ex4 NPs
3.4. In Vitro MR Images of MnMEIO and MnMEIO-Ex4 NPs and NPs-Labeled MIN6 Cells
3.5. In Vivo MR Images of MnMEIO-Ex4 NPs-Labeled Islets after Transplantation
3.6. Histological Studies of the MnMEIO-Ex4 NPs-Labeled Islet Graft
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisenbarth, G.S. Type I diabetes mellitus. A chronic autoimmune disease. N. Engl. J. Med. 1986, 314, 1360–1368. [Google Scholar] [CrossRef]
- Meloche, R.M. Transplantation for the treatment of type 1 diabetes. World J. Gastroenterol. 2007, 13, 6347–6355. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.J.; Ricordi, C.; Hering, B.J.; Auchincloss, H.; Lindblad, R.; Robertson, R.P.; Secchi, A.; Brendel, M.D.; Berney, T.; Brennan, D.C.; et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006, 355, 1318–1330. [Google Scholar] [CrossRef] [Green Version]
- McCall, M.; Shapiro, A.M. Update on islet transplantation. Cold Spring Harb. Perspect. Med. 2012, 54, 20050–20059. [Google Scholar] [CrossRef] [Green Version]
- Paty, B.W.; Bonner-Weir, S.; Laughlin, M.R.; McEwan, A.J.; Shapiro, A.M. Toward development of imaging modalities for islets after transplantation: Insights from the National Institutes of Health Workshop on Beta Cell Imaging. Transplantation 2004, 77, 1133–1137. [Google Scholar] [CrossRef] [Green Version]
- Medarova, Z.; Moore, A. MRI as a tool to monitor islet transplantation. Nat. Rev. Endocrinol. 2009, 5, 444–452. [Google Scholar] [CrossRef]
- Stepanov, A.; Burilov, V.; Pinus, M.; Mustafina, A.; Rümmeli, M.H.; Mendez, R.G.; Amirov, R.; Lukashenko, S.; Zvereva, E.; Katsuba, S.; et al. Water transverse relaxation rates in aqueous dispersions of superparamagnetic iron oxide nanoclusters with diverse hydrophilic coating. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 443, 450–458. [Google Scholar] [CrossRef]
- Fedorenko, S.; Grechkina, S.L.; Mustafina, A.R.; Kholin, K.; Stepanov, A.S.; Nizameev, I.R.; Ismaev, I.E.; Kadirov, M.K.; Zairov, R.R.; Fattakhova, A.N.; et al. Tuning the non-covalent confinement of Gd (III) complexes in silica nanoparticles for high T1-weighted MR imaging capability. Colloids Surf. B Biointerfaces 2017, 149, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, S.; Stepanov, A.; Zairov, R.; Kaman, O.; Amirov, R.; Nizameev, I.; Kholin, K.; Ismaev, I.; Voloshina, A.; Sapunova, A.; et al. One-pot embedding of iron oxides and Gd(III) complexes into silica nanoparticles—Morphology and aggregation effects on MRI dual contrasting ability. Colloids Surf. A 2018, 559, 60–67. [Google Scholar] [CrossRef]
- Evgenov, N.V.; Medarova, Z.; Dai, G.; Bonner-Weir, S.; Moore, A. In vivo imaging of islet transplantation. Nat. Med. 2006, 12, 144–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evgenov, N.V.; Medarova, Z.; Pratt, J.; Pantazopoulos, P.; Leyting, S.; Bonner-Weir, S.; Moore, A. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes 2006, 55, 2419–2428. [Google Scholar] [CrossRef] [Green Version]
- Evgenov, N.V.; Pratt, J.; Pantazopoulos, P.; Moore, A. Effects of glucose toxicity and islet purity on in vivo magnetic resonance imaging of transplanted pancreatic islets. Transplantation 2008, 85, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Hathout, E.; Chan, N.K.; Tan, A.; Sakata, N.; Mace, J.; Pearce, W.; Peverini, R.; Chinnock, R.; Sowers, L.; Obenaus, A. In vivo imaging demonstrates a time-line for new vessel formation in islet transplantation. Pediatr. Transplant. 2009, 13, 892–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jirák, D.; Kríz, J.; Herynek, V.; Andersson, B.; Girman, P.; Burian, M.; Saudek, F.; Hájek, M. MRI of transplanted pancreatic islets. Magn. Reson. Med. 2004, 52, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Berkova, Z.; Kriz, J.; Girman, P.; Zacharovova, K.; Koblas, T.; Dovolilova, E.; Saudek, F. Vitality of pancreatic islets labeled for magnetic resonance imaging with iron particles. Transplant. Proc. 2005, 37, 3496. [Google Scholar] [CrossRef]
- Kriz, J.; Jirák, D.; Girman, P.; Berková, Z.; Zacharovova, K.; Honsova, E.; Lodererova, A.; Hajek, M.; Saudek, F. Magnetic resonance imaging of pancreatic islets in tolerance and rejection. Transplantation 2005, 80, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.H.; Foster, P.; Rosales, A.; Moore, A. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes 2006, 55, 2931–2938. [Google Scholar] [CrossRef] [Green Version]
- Berkova, Z.; Jirak, D.; Zacharovova, K.; Kriz, J.; Lodererova, A.; Girman, P.; Koblas, T.; Dovolilova, E.; Vancova, M.; Hajek, M.; et al. Labeling of pancreatic islets with iron oxide nanoparticles for in vivo detection with magnetic resonance. Transplantation 2008, 85, 155–159. [Google Scholar] [CrossRef]
- Jiao, Y.; Peng, Z.H.; Xing, T.H.; Qin, J.; Zhong, C.P. Assessment of islet graft survival using a 3.0-Tesla magnetic resonance scanner. Anat. Rec. 2008, 291, 1684–1692. [Google Scholar] [CrossRef]
- Zhang, S.; He, H.; Lu, W.; Xu, Q.; Zhou, B.; Tang, X. Tracking intrahepatically transplanted islets labeled with Feridex-polyethyleneimine complex using a clinical 3.0-T magnetic resonance imaging scanner. Pancreas 2009, 38, 293–302. [Google Scholar] [CrossRef]
- Marzola, P.; Longoni, B.; Szilagyi, E.; Merigo, F.; Nicolato, E.; Fiorini, S.; Paoli, G.T.; Benati, D.; Mosca, F.; Sbarbati, A. In vivo visualization of transplanted pancreatic islets by MRI: Comparison between in vivo, histological and electron microscopy findings. Contrast Media Mol. Imaging 2009, 4, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Medarova, Z.; Vallabhajosyula, P.; Tena, A.; Evgenov, N.; Pantazopoulos, P.; Tchipashvili, V.; Weir, G.; Sachs, D.; Moore, A. In vivo imaging of autologous islet grafts in the liver and under the kidney capsule in non-human primates. Transplantation 2009, 87, 1659–1666. [Google Scholar] [CrossRef]
- Toso, C.; Vallee, J.P.; Morel, P.; Ris, F.; Demuylder-Mischler, S.; Lepetit-Coiffe, M.; Marangon, N.; Saudek, F.; Shapiro, A.M.J.; Bosco, D.; et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am. J. Transplant. 2008, 8, 701–706. [Google Scholar] [CrossRef]
- Saudek, F.; Jirák, D.; Girman, P.; Herynek, V.; Dezortová, M.; Kríz, J.; Peregrin, J.; Berková, Z.; Zacharovová, K.; Hájek, M. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation 2010, 90, 1602–1606. [Google Scholar] [CrossRef]
- Guggenheim, E.J.; Rappoport, J.Z.; Lynch, I.; Cher, T.; Szklaruk, J. MR contrast agents: Mechanisms for cellular uptake of nanosized clinical MRI contrast agents. Nanotoxicology 2020, 14, 504–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Z.-T.; Wang, J.-F.; Kuo, H.-Y.; Shen, C.-R.; Wang, J.-J.; Yen, T.-C. In situ preparation of high relaxivity iron oxide nanoparticles by coating with chitosan: A potential MRI contrast agent useful for cell tracking. J. Magn. Magn. Mater. 2010, 322, 208–213. [Google Scholar] [CrossRef]
- Shen, C.-R.; Juang, J.-H.; Tsai, Z.-T.; Wu, S.-T.; Tsai, F.-Y.; Wang, J.-J.; Liu, C.-L.; Yen, T.-C. Preparation, characterization and application of superparamagnetic iron oxide encapsulated with N-[(2-hydroxy-3-trimethylammonium) propyl] chitosan chloride. Carbohydr. Polym. 2011, 84, 781–787. [Google Scholar] [CrossRef]
- Juang, J.-H.; Lin, H.-C.; Chen, C.-Y.; Kao, C.-W.; Chen, C.-L.; Wu, S.-T.; Lin, S.-H.; Shen, C.-R.; Wang, J.-J.; Tsai, Z.-T.; et al. Noninvasive tracking of mPEG-poly(Ala) hydrogel-embedded MIN6 cells after subcutaneous transplantation in mice. Polymers 2021, 13, 885. [Google Scholar] [CrossRef]
- Juang, J.-H.; Wang, J.-J.; Shen, C.-R.; Chen, C.-Y.; Kao, C.-W.; Chen, C.-L.; Lin, S.-H.; Wu, S.-T.; Li, W.-C.; Tsai, Z.-T. Magnetic resonance imaging of transplanted porcine neonatal pancreatic cell clusters labeled with chitosan-coated superparamagnetic iron oxide nanoparticles in mice. Polymers 2021, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Wang, J.J.; Shen, C.R.; Kuo, C.H.; Chien, Y.W.; Kuo, H.Y.; Tsai, Z.T.; Yen, T.C. Magnetic resonance imaging of transplanted mouse islets labeled with chitosan-coated superparamagnetic iron oxide nanoparticles. Transplant. Proc. 2010, 42, 2104–2108. [Google Scholar] [CrossRef]
- Juang, J.H.; Shen, C.R.; Wang, J.J.; Kuo, C.H.; Chien, Y.W.; Kuo, H.Y.; Chen, F.R.; Chen, M.H.; Yen, T.C.; Tsai, Z.T. Magnetic resonance imaging of mouse islet grafts labeled with novel chitosan-coated superparamagnetic iron oxide nanoparticles. PLoS ONE 2013, 8, e62626. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Shen, C.R.; Wang, J.J.; Kuo, C.H.; Lin, M.Y.; Wu, S.T.; Tsai, Z.T.; Yen, T.C. Magnetic resonance imaging study of mouse islet allotransplantation. Transplant. Proc. 2010, 42, 4217–4220. [Google Scholar] [CrossRef] [PubMed]
- Tornehave, D.; Kristensen, P.; Rømer, J.; Knudsen, L.B.; Heller, R.S. Expression of the GLP-1 receptor in mouse, rat, and human pancreas. J. Histochem. Cytochem. 2008, 56, 841–851. [Google Scholar] [CrossRef] [Green Version]
- Sowa-Staszczak, A.; Pach, D.; Mikolajczak, R.; Mäcke, H.; Jabrocka-Hybel, A.; Stefańska, A.; Tomaszuk, M.; Janota, B.; Gilis-Januszewska, A.; Małecki, M.; et al. Glucagon-like peptide-1 receptor imaging with [Lys(40)(Ahx-HYNIC-(99m)Tc/EDDA)NH 2]-exendin-4 for the detection of insulinoma. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Kiesewetter, D.O.; Gao, H.; Ma, Y.; Niu, G.; Quan, Q.; Guo, N.; Chen, X. 18F-radiolabeled analogs of exendin-4 for PET imaging of GLP-1 in insulinoma. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Wild, D.; Behe, M.; Wicki, A.; Storch, D.; Waser, B.; Gotthardt, M.; Keil, B.; Christofori, G.; Reubi, J.C.; Mäcke, H.R. [Lys40(Ahx-DTPA-111In)NH2]exendin-4, a very promising ligand for glucagon-like peptide-1 (GLP-1) receptor targeting. J. Nucl. Med. 2006, 47, 2025–2033. [Google Scholar]
- Christ, E.; Wild, D.; Forrer, F.; Brändle, M.; Sahli, R.; Clerici, T.; Gloor, B.; Martius, F.; Maecke, H.; Reubi, J.C. Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J. Clin. Endocrinol. Metab. 2009, 94, 4398–4405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, E.; Wild, D.; Ederer, S.; Béhé, M.; Nicolas, G.; Caplin, M.E.; Brändle, M.; Clerici, T.; Fischli, S.; Stettler, C.; et al. Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: A prospective multicentre imaging study. Lancet Diabetes Endocrinol. 2013, 1, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Pattou, F.; Kerr-Conte, J.; Wild, D. GLP-1-receptor scanning for imaging of human beta cells transplanted in muscle. N. Engl. J. Med. 2010, 363, 1289–1290. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, B.; Zhai, C.; Jiang, B.; Wu, Y. The role of exendin-4-conjugated superparamagnetic iron oxide nanoparticles in beta-cell-targeted MRI. Biomaterials 2013, 34, 5843–5852. [Google Scholar] [CrossRef]
- Wang, P.; Yoo, B.; Yang, J.; Zhang, X.; Ross, A.; Pantazopoulos, P.; Dai, G.; Moore, A. GLP-1R-targeting magnetic nanoparticles for pancreatic islet imaging. Diabetes 2014, 63, 1465–1474. [Google Scholar] [CrossRef] [Green Version]
- Vinet, L.; Lamprianou, S.; Babič, A.; Lange, N.; Thorel, F.; Herrera, P.L.; Montet, X.; Meda, P. Targeting GLP-1 receptors for repeated magnetic resonance imaging differentiates graded losses of pancreatic beta cells in mice. Diabetologia 2015, 58, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Schmieder, A.H.; Wickline, S.A.; Lanza, G.M. Manganese-based MRI contrast agents: Past, present and future. Tetrahedron 2011, 67, 8431–8444. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Huh, Y.M.; Jun, Y.W.; Seo, J.W.; Jang, J.T.; Song, H.T.; Kim, S.; Cho, E.J.; Yoon, H.G.; Suh, J.S.; et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Lin, K.L.; Wang, T.P.; Tzou, S.C.; Singh, G.; Chen, M.H.; Cheng, T.L.; Chen, C.Y.; Liu, G.C.; Lee, T.W.; et al. Imaging specificity of MR-optical imaging agents following the masking of surface charge by poly(ethylene glycol). Imaging specificity of MR-optical imaging agents following the masking of surface charge by poly(ethylene glycol). Biomaterials 2013, 34, 4118–4127. [Google Scholar] [CrossRef]
- Wu, S.-C.; Chen, Y.-J.; Lin, Y.-J.; Wu, T.-H.; Wang, Y.-M. Development of a mucin4-targeting SPIO contrast agent for effective detection of pancreatic tumor cells in vitro and in vivo. J. Med. Chem. 2013, 56, 9100–9109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Yang, X.; Fan, F.; Li, Y. Factors affecting the determination of iron species in the presence of ferric iron. Appl. Water Sci. 2018, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Juang, J.H.; Bonner-Weir, S.; Wu, Y.J.; Weir, G.C. Beneficial influence of glycemic control upon the growth and function of transplanted islets. Diabetes 1994, 43, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Ma, Z.; Khor, E.; Lim, L.Y. Uptake of FITC-chitosan nanoparticles by A549 cells. Pharm. Res. 2002, 19, 1488–1494. [Google Scholar] [CrossRef]














| Materials | Hydrodynamic Size (nm) | Zeta Potential (mV) |
|---|---|---|
| MnMEIO NPs | 24.2 ± 2.3 | −5.1 ± 0.3 |
| MnMEIO (MnMEIO-silane-NH2-mPEG) | 67.8 ± 1.3 | 33.3 ± 0.5 |
| MnMEIO-Ex4 (MnMEIO-silane-NH2-mPEG-Ex4) | 70.2 ± 2.3 | 0.6 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juang, J.-H.; Shen, C.-R.; Wang, J.-J.; Wu, S.-T.; Lin, S.-H.; Chen, C.-Y.; Kao, C.-W.; Chen, C.-L.; Tsai, Z.-T.; Wang, Y.-M. Exendin-4-Conjugated Manganese Magnetism-Engineered Iron Oxide Nanoparticles as a Potential Magnetic Resonance Imaging Contrast Agent for Tracking Transplanted β-Cells. Nanomaterials 2021, 11, 3145. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11113145
Juang J-H, Shen C-R, Wang J-J, Wu S-T, Lin S-H, Chen C-Y, Kao C-W, Chen C-L, Tsai Z-T, Wang Y-M. Exendin-4-Conjugated Manganese Magnetism-Engineered Iron Oxide Nanoparticles as a Potential Magnetic Resonance Imaging Contrast Agent for Tracking Transplanted β-Cells. Nanomaterials. 2021; 11(11):3145. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11113145
Chicago/Turabian StyleJuang, Jyuhn-Huarng, Chia-Rui Shen, Jiun-Jie Wang, Shu-Ting Wu, Sung-Han Lin, Chen-Yi Chen, Chen-Wei Kao, Chen-Ling Chen, Zei-Tsan Tsai, and Yun-Ming Wang. 2021. "Exendin-4-Conjugated Manganese Magnetism-Engineered Iron Oxide Nanoparticles as a Potential Magnetic Resonance Imaging Contrast Agent for Tracking Transplanted β-Cells" Nanomaterials 11, no. 11: 3145. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11113145