Cysteine-Functionalized Chitosan Magnetic Nano-Based Particles for the Recovery of Light and Heavy Rare Earth Metals: Uptake Kinetics and Sorption Isotherms
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Sorbent Particles
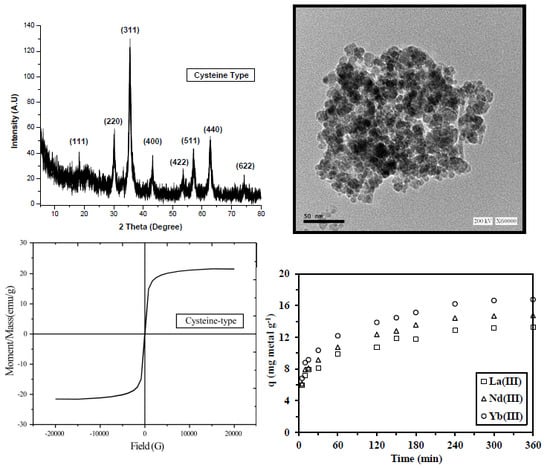
2.2. Characterization of Sorbents





2.3. Sorption Properties
2.3.1. Sorption as a Function of pH

2.3.2. Uptake Kinetics

| Metal ion | qeq., exp. (mg·g−1) | PFORE | PSORE | ||||
|---|---|---|---|---|---|---|---|
| k1 × 102 (min−1) | qe (mg/g) | R2 | k2 × 103 (g·mg−1·min−1) | qe (mg/g) | R2 | ||
| La(III) | 13.3 | 1.04 | 6.37 | 0.960 | 4.2 | 13.68 | 0.995 |
| Nd(III) | 14.8 | 1.15 | 7.75 | 0.984 | 3.7 | 15.27 | 0.996 |
| Yb(III) | 16.8 | 1.13 | 8.64 | 0.990 | 3.1 | 17.33 | 0.996 |
| Metal ion | Intraparticle diffusion | Elovich equation | ||||
|---|---|---|---|---|---|---|
| c, mg·g−1 | kint., mg·g−1·min−0.5 | R2 | BE | AT | R2 | |
| La(III) | 4.26 | 0.56 | 0.832 | 1.75 | 2.90 | 0.979 |
| Nd(III) | 4.49 | 0.64 | 0.850 | 2.07 | 2.64 | 0.988 |
| Yb(III) | 5.02 | 0.86 | 0.855 | 2.36 | 2.86 | 0.991 |
2.3.3. Sorption Isotherms

| Metal ion | T (K) | qm.,exp. (mg·g−1) | Langmuir model | Freundlich model | ||||
|---|---|---|---|---|---|---|---|---|
| qm.,calc. | KL (L·mg−1) | R2 | n | KF, (mg·g−1) | R2 | |||
| La(III) | 300 | 15.0 | 16.0 | 0.071 | 0.997 | 0.20 | 5.27 | 0.921 |
| 310 | 16.2 | 17.1 | 0.079 | 0.998 | 0.20 | 5.84 | 0.953 | |
| 320 | 17.0 | 17.9 | 0.086 | 0.998 | 0.19 | 6.46 | 0.971 | |
| Nd(III) | 300 | 15.3 | 16.0 | 0.106 | 0.999 | 0.19 | 5.92 | 0.934 |
| 310 | 16.3 | 16.8 | 0.131 | 0.999 | 0.180 | 6.64 | 0.918 | |
| 320 | 17.1 | 17.6 | 0.144 | 0.999 | 0.17 | 7.17 | 0.920 | |
| Yb(III) | 300 | 17.8 | 18.7 | 0.086 | 0.998 | 0.21 | 6.00 | 0.890 |
| 310 | 18.1 | 18.9 | 0.120 | 0.998 | 0.18 | 7.40 | 0.871 | |
| 320 | 18.4 | 19.3 | 0.154 | 0.999 | 0.16 | 8.20 | 0.913 | |
| Metal ion | T (K) | qm (mg·g−1) | D–R Isotherm model | Temkin model | ||||
|---|---|---|---|---|---|---|---|---|
| Kad × 104 (mol2·kJ−2) | EDR (KJ·mol−1) | R2 | AT (L·mg−1) | BT (J·mol−1) | R2 | |||
| La(III) | 300 | 15.0 | 0.8 | 0.079 | 0.994 | 3.94 | 2.25 | 0.914 |
| 310 | 16.2 | 0.6 | 0.091 | 0.939 | 5.00 | 2.35 | 0.945 | |
| 320 | 17.0 | 0.5 | 0.100 | 0.944 | 7.18 | 2.34 | 0.962 | |
| Nd(III) | 300 | 15.3 | 1.0 | 0.071 | 0.968 | 6.67 | 2.19 | 0.948 |
| 310 | 16.3 | 0.7 | 0.085 | 0.973 | 10.45 | 2.20 | 0.950 | |
| 320 | 17.1 | 0.5 | 0.100 | 0.981 | 13.62 | 2.23 | 0.955 | |
| Yb(III) | 300 | 17.8 | 0.6 | 0.091 | 0.997 | 3.36 | 2.79 | 0.902 |
| 310 | 18.1 | 0.4 | 0.112 | 0.997 | 10.41 | 2.44 | 0.876 | |
| 320 | 18.4 | 0.2 | 0.158 | 0.994 | 20.52 | 2.28 | 0.918 | |
2.3.4. Effect of Temperature—Thermodynamic Studies
| Metal ion | T (K) | ∆H° (kJ·mol−1) | ∆S° (kJ·mol−1) | ∆G° (kJ·mol−1) | T∆S° (kJ·mol−1) | R2 |
|---|---|---|---|---|---|---|
| La(III) | 300 | 7.85 | 0.103 | −22.94 | 30.8 | 0.998 |
| 310 | −23.97 | 31.8 | ||||
| 320 | −24.99 | 32.8 | ||||
| Nd(III) | 300 | 12.04 | 0.120 | −24.01 | 36.1 | 0.957 |
| 310 | −25.29 | 37.3 | ||||
| 320 | −26.49 | 38.5 | ||||
| Yb(III) | 300 | 23.43 | 0.158 | −23.99 | 47.4 | 0.995 |
| 310 | −25.57 | 49.0 | ||||
| 320 | −27.15 | 50.6 |

| Sorbent | Metal | pH range | qm (mg·g−1) | Reference |
|---|---|---|---|---|
| Tangerine peel | La(III) | 5 | 155 | [52] |
| Sargassum sp. | La(III) | 5 | 74–100 | [53] |
| Magnetic alginate beads | La(III) | 2.8 | 97 | [16] |
| Platanus orientalis leaf | La(III) | 4 | 29 | [54] |
| Lewatit resins | La(III) | 1.5–5 | 100–120 | [55] |
| Chelating ion-exchange resin | La(III) | HCl/HNO3 | 188–240 | [56] |
| Functionalized Amberlite XAD-4 resin | La(III) | 6.1 | 49 | [57] |
| CFCMNBP | La(III) | 6 | 17 | This work |
| EDTA:DTPA functionalized chitosan | Nd(III) | 3–6 | 55 | [10] |
| Phosphonic acid functionalized silica microspheres | Nd(III) | 2.8 | 45 | [58] |
| Ion imprinted polymer particles | Nd(III) | 7.5 | 33 | [59] |
| Phosphorus functionalized adsorbent | Nd(III) | 6 | 160 | [60] |
| Yeast cells | Nd(III) | 1.5 | 10–12 | [61] |
| Mordenite containing tuff | Nd(III) | 5.5–6.5 | 13 | [62] |
| CFCMNBP | Nd(III) | 6 | 17 | This work |
| Sargassum | Yb(III) | 5 | 160 | [27] |
| Turbinaria conoides | Yb(III) | 4.9 | 34 | [63] |
| Pseudomonas aeruginosa | Yb(III) | 6–7 | 56 | [64] |
| Imino-diacetic acid resin | Yb(III) | 5.1 | 187 | [65] |
| Gel-type weak acid resin | Yb(III) | 5.5 | 266 | [66] |
| CFCMNBP | Yb(III) | 6 | 18 | This work |
2.3.5. Metal Desorption and Resin Recycling
| Cycle | La(III) | Nd(III) | Yb(III) | |||
|---|---|---|---|---|---|---|
| qe (mg·g−1) | Ads. (%) | qe (mg·g−1) | Ads. (%) | qe (mg·g−1) | Ads. (%) | |
| Cycle I | 12.9 | 100.0 | 14.4 | 100.0 | 16.2 | 100.0 |
| Cycle II | 12.7 | 98.2 | 14.2 | 98.1 | 15.4 | 95.2 |
| Cycle III | 12.6 | 97.5 | 14.1 | 97.5 | 15.3 | 94.3 |
| Cycle IV | 12.6 | 97.1 | 14.0 | 96.9 | 15.2 | 93.8 |
2.3.6. Sorption Selectivity
3. Experimental Section
3.1. Materials
3.2. Rare Earth Solutions and Analytical Procedures
3.3. Sorbent Synthesis and Characterization
3.3.1. Preparation of Cross-Linked Chitosan-Magnetite Nanocomposites
3.3.2. Characterization Methods
3.3.3. Sorption and Desorption Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, X.; Jiao, C.; Wang, J.; Liu, Q.; Li, R.; Yang, P.; Zhang, M. Removal of uranium(VI) from aqueous solutions by magnetic schiff base: Kinetic and thermodynamic investigation. Chem. Eng. J. 2012, 198, 412–419. [Google Scholar] [CrossRef]
- Namdeo, M.; Bajpai, S.K. Chitosan-magnetite nanocomposites (CMNs) as magnetic carrier particles for removal of Fe(III) from aqueous solutions. Colloids Surf. A 2008, 320, 161–168. [Google Scholar] [CrossRef]
- Wang, G.H.; Liu, J.S.; Wang, X.G.; Xie, Z.Y.; Deng, N.S. Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J. Hazard. Mater. 2009, 168, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Hosoba, M.; Oshita, K.; Katarina, R.K.; Takayanagi, T.; Oshima, M.; Motomizu, S. Synthesis of novel chitosan resin possessing histidine moiety and its application to the determination of trace silver by ICP-AES coupled with triplet automated-pretreatment system. Anal. Chim. Acta 2009, 639, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Oshita, K.; Takayanagi, T.; Oshima, M.; Motomizu, S. Adsorption behavior of cationic and anionic species on chitosan resins possessing amino acid moieties. Anal. Sci. 2007, 23, 1431–1434. [Google Scholar] [CrossRef] [PubMed]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Gao, Y.H.; Oshita, K.; Lee, K.H.; Oshima, M.; Motomizu, S. Development of column-pretreatment chelating resins for matrix elimination/multi-element determination by inductively coupled plasma-mass spectrometry. Analyst 2002, 127, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-S.; Peng, R.-T.; Yang, J.-H.; Liu, Y.-C.; Hu, X.-J. Preparation of ethylenediamine-modified magnetic chitosan complex for adsorption of uranyl ions. Carbohydr. Polym. 2011, 84, 1169–1175. [Google Scholar] [CrossRef]
- Oshita, K.; Sabarudin, A.; Takayanagi, T.; Oshima, M.; Motomizu, S. Adsorption behavior of uranium(VI) and other ionic species on cross-linked chitosan resins modified with chelating moieties. Talanta 2009, 79, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Roosen, J.; Binnemans, K. Adsorption and chromatographic separation of rare earths with edta- and dtpa-functionalized chitosan biopolymers. J. Mater. Chem. A 2014, 2, 1530–1540. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan—Present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Xu, J.; Liang, X.; Liu, Z. Adsorption of platinum(IV) and palladium(II) from aqueous solution by magnetic cross-linking chitosan nanoparticles modified with ethylenediamine. J. Hazard. Mater. 2010, 182, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Wang, J.; Mei, L.; Wang, Z.; Qi, K.; Yang, B. Recognition and enrichment specificity of Fe3O4 magnetic nanoparticles surface modified by chitosan and Staphylococcus aureus enterotoxins a antiserum. Colloids Surf. B 2013, 103, 107–113. [Google Scholar] [CrossRef]
- Karaca, E.; Şatır, M.; Kazan, S.; Açıkgöz, M.; Öztürk, E.; Gürdağ, G.; Ulutaş, D. Synthesis, characterization and magnetic properties of Fe3O4 doped chitosan polymer. J. Magn. Magn. Mater. 2015, 373, 53–59. [Google Scholar] [CrossRef]
- Zhou, Z.; Lin, S.; Yue, T.; Lee, T.-C. Adsorption of food dyes from aqueous solution by glutaraldehyde cross-linked magnetic chitosan nanoparticles. J. Food Eng. 2014, 126, 133–141. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, L.; Wang, L.; Zhu, B.; Fan, L. Adsorption of lanthanum by magnetic alginate-chitosan gel beads. J. Chem. Technol. Biotechnol. 2011, 86, 345–352. [Google Scholar] [CrossRef]
- Dodi, G.; Hritcu, D.; Lisa, G.; Popa, M.I. Core-shell magnetic chitosan particles functionalized by grafting: Synthesis and characterization. Chem. Eng. J. 2012, 203, 130–141. [Google Scholar] [CrossRef]
- Dupont, D.; Brullot, W.; Bloemen, M.; Verbiest, T.; Binnemans, K. Selective uptake of rare earths from aqueous solutions by edta-functionalized magnetic and nonmagnetic nanoparticles. ACS Appl. Mater. Interface 2014, 6, 4980–4988. [Google Scholar] [CrossRef]
- Cowan, C.E.; Zachara, J.M.; Resch, C.T. Cadmium adsorption on iron-oxides in the presence of alkaline-earth elements. Environ. Sci. Technol. 1991, 25, 437–446. [Google Scholar] [CrossRef]
- Trivedi, P.; Axe, L. Modeling Cd and Zn sorption to hydrous metal oxides. Environ. Sci. Technol. 2000, 34, 2215–2223. [Google Scholar] [CrossRef]
- Koeppenkastrop, D.; Decarlo, E.H. Uptake of rare-earth elements from solution by metal-oxides. Environ. Sci. Technol. 1993, 27, 1796–1802. [Google Scholar] [CrossRef]
- Zhou, J.; Duan, W.; Zhou, X.; Zhang, C. Application of annular centrifugal contactors in the extraction flowsheet for producing high purity yttrium. Hydrometallurgy 2007, 85, 154–162. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Elsevier Butterworth-Heinemann: Oxford, UK, 1997; p. 1305. [Google Scholar]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006; p. 263. [Google Scholar]
- Martins, T.S.; Isolani, P.C. Rare earths: Industrial and biological applications. Quim. Nova 2005, 28, 111–117. [Google Scholar] [CrossRef]
- Pearson, R.G. Acids and bases. Science 1966, 151, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Diniz, V.; Volesky, B. Biosorption of La, Eu and Yb using Sargassum biomass. Water Res. 2005, 39, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Donia, A.M.; Atia, A.A.; Daher, A.M.; Desouky, O.A.; Elshehy, E.A. Synthesis of amine/thiol magnetic resin and study of its interaction with Zr(IV) and Hf(IV) ions in their aqueous solutions. J. Dispers. Sci. Technol. 2011, 32, 634–641. [Google Scholar] [CrossRef]
- Filha, V.; Wanderley, A.F.; de Sousa, K.S.; Espinola, J.G.P.; da Fonseca, M.G.; Arakaki, T.; Arakaki, L.N.H. Thermodynamic properties of divalent cations complexed by ethylenesulfide immobilized on silica gel. Colloids Surf. A 2006, 279, 64–68. [Google Scholar] [CrossRef]
- Chethan, P.D.; Vishalakshi, B. Synthesis of ethylenediamine modified chitosan and evaluation for removal of divalent metal ions. Carbohydr. Polym. 2013, 97, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Agnely, F.; Leclerc, B.; Siepmann, J.; Cotte, M.; Geiger, S.; Couarraze, G. Cross-linking of chitosan and chitosan/poly(ethylene oxide) beads: A theoretical treatment. Eur. J. Pharm. Biopharm. 2007, 67, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.L.; Laranjeira, M.C.M.; Fávere, V.T.; Pedrosa, R.C. Effect of crosslinking agents on chitosan microspheres in controlled release of diclofenac sodium. Polim. Cienc. Tecnol. 2005, 15, 6–12. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Guinier, A.; Lorrain, P.; Lorrain, D.S.-M. X-Ray Diffraction: In Crystals, Imperfect Crystals and Amorphous Bodies; W.H. Freeman & Co.: San Francisco, CA, USA, 1963; p. 356. [Google Scholar]
- Salazar-Camacho, C.; Villalobos, M.; Rivas-Sánchez, M.D.L.L.; Arenas-Alatorre, J.; Alcaraz-Cienfuegos, J.; Gutiérrez-Ruiz, M.E. Characterization and surface reactivity of natural and synthetic magnetites. Chem. Geol. 2013, 347, 233–245. [Google Scholar] [CrossRef]
- Kim, D.-H.; Nikles, D.E.; Brazel, C.S. Synthesis and characterization of multifunctional chitosan-MnFe2O4 nanoparticles for magnetic hyperthermia and drug delivery. Materials 2010, 3, 4051–4065. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J. Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu(II) removal. Chem. Eng. J. 2011, 168, 286–292. [Google Scholar] [CrossRef]
- Yan, H.; Li, H.; Yang, H.; Li, A.; Cheng, R. Removal of various cationic dyes from aqueous solutions using a kind of fully biodegradable magnetic composite microsphere. Chem. Eng. J. 2013, 223, 402–411. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Lee, S.-M. Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv. Colloid Interface Sci. 2013, 201, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Deliyanni, E.A. Mercury(II) removal with modified magnetic chitosan adsorbents. Molecules 2013, 18, 6193–6214. [Google Scholar] [CrossRef] [PubMed]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.; Zhang, W.; Zhang, Q. Review: Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Anagnostopoulos, V.A.; Symeopoulos, B.D. Sorption of europium by malt spent rootlets, a low cost biosorbent: Effect of pH, kinetics and equilibrium studies. J. Radioanal. Nucl. Chem. 2013, 295, 7–13. [Google Scholar] [CrossRef]
- Hu, X.-J.; Wang, J.-S.; Liu, Y.-G.; Li, X.; Zeng, G.-M.; Bao, Z.-L.; Zeng, X.-X.; Chen, A.-W.; Long, F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, L.; Cao, K.; Geng, J.; Liu, J.; Song, Q.; Yang, X.; Li, S. Selective solid-phase extraction of uranium by salicylideneimine-functionalized hydrothermal carbon. J. Hazard. Mater. 2012, 229, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Amer. Chem. Soc. 1918, 40, 1361–1402. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Temkin, V.P. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. 1940, 12, 217–222. [Google Scholar]
- Freundlich, H.M.F. Uber die adsorption in lasungen. Z. Phys. Chem. 1906, 57, 385–470. (In German) [Google Scholar]
- Dubinin, M.M.; Zaverina, E.D.; Radushkevich, L.V. Sorption and structure of active carbons. I. Adsorption of organic vapors. Zh. Fiz. Khim. 1947, 21, 1351–1362. [Google Scholar]
- Rahmati, A.; Ghaemi, A.; Samadfam, M. Kinetic and thermodynamic studies of uranium(VI) adsorption using amberlite ira-910 resin. Ann. Nucl. Energy 2012, 39, 42–48. [Google Scholar] [CrossRef]
- Torab-Mostaedi, M. Biosorption of lanthanum and cerium from aqueous solutions using tangerine (citrus reticulate) peel: Equilibrium, kinetic and thermodynamic studies. Chem. Ind. Chem. Eng. Q. 2013, 19, 79–88. [Google Scholar] [CrossRef]
- Palmieri, M.C.; Volesky, B.; Garcia, O. Biosorption of lanthanum using sargassum fluitans in batch system. Hydrometallurgy 2002, 67, 31–36. [Google Scholar] [CrossRef]
- Sert, Ş.; Kütahyali, C.; İnan, S.; Talip, Z.; Çetinkaya, B.; Eral, M. Biosorption of lanthanum and cerium from aqueous solutions by platanus orientalis leaf powder. Hydrometallurgy 2008, 90, 13–18. [Google Scholar] [CrossRef]
- Esma, B.; Omar, A.; Amine, D.M. Comparative study on lanthanum(III) sorption onto lewatit TP 207 and lewatit TP 260. J. Radioanal. Nucl. Chem. 2014, 299, 439–446. [Google Scholar] [CrossRef]
- Maheswari, M.A.; Subramanian, M.S. Selective enrichment of U(VI), Th(IV) and La(III) from high acidic streams using a new chelating ion-exchange polymeric matrix. Talanta 2004, 64, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Dev, K.; Pathak, R.; Rao, G.N. Sorption behaviour of lanthanum(III), neodymium(III), terbium(III), thorium(IV) and uranium(VI) on Amberlite XAD-4 resin functionalized with bicine ligands. Talanta 1999, 48, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, I.V.; Goncharyk, V.P.; Kozhara, L.I.; Yurchenko, G.R.; Matkovsky, A.K.; Zub, Y.L.; Alonso, B. Sorption properties of porous spray-dried microspheres functionalized by phosphonic acid groups. Microporous Mesoporous Mater. 2012, 153, 171–177. [Google Scholar] [CrossRef]
- Krishna, P.G.; Gladis, J.M.; Rao, T.P.; Naidu, G.R. Selective recognition of neodymium(III) using ion imprinted polymer particles. J. Mol. Recognit. 2005, 18, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Tavlarides, L.L. Adsorption of neodymium(III) from aqueous solutions using a phosphorus functionalized adsorbent. Ind. Eng. Chem. Res. 2010, 49, 12567–12575. [Google Scholar] [CrossRef]
- Vlachou, A.; Symeopoulos, B.D.; Koutinas, A.A. A comparative study of neodymium sorption by yeast cells. Radiochim. Acta 2009, 97, 437–441. [Google Scholar]
- Kozhevnikova, N.M.; Tsybikova, N.L. Sorption of neodymium(III) ions by natural mordenite-containing tuff. Russ. J. Appl. Chem. 2008, 81, 42–45. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Sathishkumar, M.; Balasubramanian, R. Interaction of rare earth elements with a brown marine alga in multi-component solutions. Desalination 2011, 265, 54–59. [Google Scholar] [CrossRef]
- Texier, A.C.; Andres, Y.; le Cloirec, P. Selective biosorption of lanthanide (La, Eu, Yb) ions by pseudomonas aeruginosa. Environ. Sci. Technol. 1999, 33, 489–495. [Google Scholar] [CrossRef]
- Xiong, C.; Yao, C.; Wang, Y. Sorption behaviour and mechanism of ytterbium(III) on imino-diacetic acid resin. Hydrometallurgy 2006, 82, 190–194. [Google Scholar] [CrossRef]
- Zheng, Z.; Xiong, C. Adsorption behavior of ytterbium (III) on gel-type weak acid resin. J. Rare Earths 2011, 29, 407–412. [Google Scholar] [CrossRef]
- Konishi, Y.; Shimaoka, J.-I.; Asai, S. Sorption of rare-earth ions on biopolymer gel beads of alginic acid. React. Funct. Polym. 1998, 36, 197–206. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Stetzenbach, K.J.; Hodge, V.F.; Lyons, W.B. Rare earth element complexation behavior in circumneutral pH groundwaters: Assessing the role of carbonate and phosphate ions. Earth Planet. Sci. Lett. 1996, 139, 305–319. [Google Scholar] [CrossRef]
- Piasecki, W.; Sverjensky, D.A. Speciation of adsorbed yttrium and rare earth elements on oxide surfaces. Geochim. Cosmochim. Acta 2008, 72, 3964–3979. [Google Scholar] [CrossRef]
- Schijf, J.; Marshall, K.S. Yree sorption on hydrous ferric oxide in 0.5 M NaCl solutions: A model extension. Mar. Chem. 2011, 123, 32–43. [Google Scholar] [CrossRef]
- Tang, J.W.; Johannesson, K.H. Adsorption of rare earth elements onto carrizo sand: Experimental investigations and modeling with surface complexation. Geochim. Cosmochim. Acta 2005, 69, 5247–5261. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Endud, C.S.; Mayanar, R. Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar]
- Donia, A.M.; Atia, A.A.; Abouzayed, F.I. Preparation and characterization of nano-magnetic cellulose with fast kinetic properties towards the adsorption of some metal ions. Chem. Eng. J. 2012, 191, 22–30. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galhoum, A.A.; Mafhouz, M.G.; Abdel-Rehem, S.T.; Gomaa, N.A.; Atia, A.A.; Vincent, T.; Guibal, E. Cysteine-Functionalized Chitosan Magnetic Nano-Based Particles for the Recovery of Light and Heavy Rare Earth Metals: Uptake Kinetics and Sorption Isotherms. Nanomaterials 2015, 5, 154-179. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5010154
Galhoum AA, Mafhouz MG, Abdel-Rehem ST, Gomaa NA, Atia AA, Vincent T, Guibal E. Cysteine-Functionalized Chitosan Magnetic Nano-Based Particles for the Recovery of Light and Heavy Rare Earth Metals: Uptake Kinetics and Sorption Isotherms. Nanomaterials. 2015; 5(1):154-179. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5010154
Chicago/Turabian StyleGalhoum, Ahmed A., Mohammad G. Mafhouz, Sayed T. Abdel-Rehem, Nabawia A. Gomaa, Asem A. Atia, Thierry Vincent, and Eric Guibal. 2015. "Cysteine-Functionalized Chitosan Magnetic Nano-Based Particles for the Recovery of Light and Heavy Rare Earth Metals: Uptake Kinetics and Sorption Isotherms" Nanomaterials 5, no. 1: 154-179. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5010154