2D In-Plane CuS/Bi2WO6 p-n Heterostructures with Promoted Visible-Light-Driven Photo-Fenton Degradation Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Sample Preparation
2.2.1. Preparation of Bi2WO6 Nanosheets
2.2.2. Preparation of CuS/Bi2WO6 Heterostructure
2.3. Characterization
2.4. Photocatalytic and Photo-Fenton Catalytic Activity Measurement
3. Results and Discussion
3.1. Structure, Composition, and Morphology of Samples
3.2. Photocatalytic and Photo-Fenton Catalytic Performance
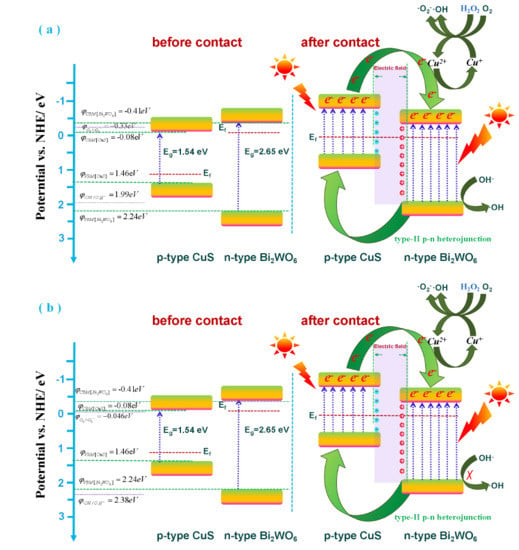
3.3. Photocatalytic and Photo-Fenton Catalytic Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, M.; Tan, G.Q.; Zhang, D.; Li, B.; Lv, L.; Wang, Y.; Ren, H.J.; Zhang, X.L.; Xia, A.; Liu, Y. Defect-mediated Z-scheme BiO2-x/Bi2O2.75 Photocatalyst for Full Spectrum Solar-Driven Organic Dyes Degradation. Appl. Catal. B: Environ. 2019, 254, 98–112. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions-A Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. In Situ Fabrication of SnO2/S-doped g-C3N4 Nanocomposites and Improved Visible Light Driven Photodegradation of Methylene Blue. J. Mol. Liquid 2017, 248, 688–702. [Google Scholar] [CrossRef]
- Pereira, J.H.O.S.; Vilar, V.J.P.; Borges, M.T.; González, O.; Esplugas, S.; Boaventura, R.A.R. Photocatalytic Degradation of Oxytetracycline using TiO2 under Natural and Simulated Solar Radiation. Sol. Energy 2011, 85, 2732–2740. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline Antibiotics in the Environment: A Review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Feng, B.; Wu, Z.Y.; Liu, J.S.; Zhu, K.J.; Li, Z.Q.; Jin, X.; Hou, Y.D.; Xi, Q.Y.; Cong, M.Q.; Liu, P.C.; et al. Combination of Ultrafast Dye-Sensitized-Assisted Electron Transfer Process and Novel Z-Scheme System: AgBr Nanoparticles Interspersed MoO3 Nanobelts for Enhancing Photocatalytic Performance of RhB. Appl. Catal. B: Environ. 2017, 206, 242–251. [Google Scholar] [CrossRef]
- Shao, B.B.; Liu, X.J.; Liu, Z.F.; Zeng, G.M.; Liang, Q.H.; Liang, C.; Cheng, Y.; Zhang, W.; Liu, Y.; Gong, S.X. A Novel Double Z-Scheme Photocatalyst Ag3PO4/Bi2S3/Bi2O3 with Enhanced Visible-Light Photocatalytic Performance for Antibiotic Degradation. Chem. Eng. J. 2019, 368, 730–745. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced Oxidation Processes (AOP) for Water Purification and Recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Giannakis, S.; Liu, S.T.; Carratalà, A.; Rtimi, S.; Bensimon, M.; Pulgarin, C. Effect of Fe(II)/Fe(III) Species, pH, Irradiance and Bacterial Presence on Viral Inactivation in Wastewater by the Photo-Fenton Process: Kinetic Modeling and Mechanistic Interpretation. Appl. Catal. B: Environ. 2017, 204, 156–166. [Google Scholar] [CrossRef]
- Li, W.H.; Wu, X.F.; Li, S.D.; Tang, W.X.; Chen, Y.F. Magnetic Porous Fe3O4/Carbon Octahedra Derived from Iron-based Metal-Organic Framework as Heterogeneous Fenton-like Catalyst. Appl. Surf. Sci. 2018, 436, 252–262. [Google Scholar] [CrossRef]
- Nogueira, R.F.P.; Oliveira, M.C.; Paterlini, W.C. Simple and Fast Spectrophotometric Determination of H2O2 in Photo-Fenton Reactions Using Metavanadate. Talanta 2005, 66, 86–91. [Google Scholar] [CrossRef]
- Jiang, J.J.; Gao, J.Y.; Li, T.R.; Chen, Y.F.; Wu, Q.N.; Xie, T.T.; Lin, Y.H.; Dong, S.S. Visible-Light-Driven Photo-Fenton Reaction with α-Fe2O3/BiOI at Near Neutral pH: Boosted Photogenerated Charge Separation, Optimum Operating Parameters and Mechanism Insight. J. Colloid Interface Sci. 2019, 554, 531–543. [Google Scholar] [CrossRef]
- Jiang, L.L.; Zhang, L.; Cui, C.; Zhang, J.; Liu, G.D.; Song, J.J. Efficient Degradation of Phenol using Sn4+ Doped FeOCl as Photo-Fenton Catalyst. Mater. Lett. 2019, 240, 30–34. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, B.D.; Zhang, G.Y.; Gan, Y.H.; Zhang, S.J. Enhanced Decomplexation of Cu(II)-EDTA: The Role of Acetylacetone in Cu-Mediated Photo-Fenton Reactions. Chem. Eng. J. 2019, 358, 1218–1226. [Google Scholar] [CrossRef]
- Xu, Z.; Shan, C.; Xie, B.H.; Liu, Y.; Pan, B.C. Decomplexation of Cu(II)-EDTA by UV/Persulfate and UV/H2O2: Efficiency and Mechanism. Appl. Catal. B: Environ. 2017, 200, 439–447. [Google Scholar] [CrossRef]
- Xu, T.Y.; Zhu, R.L.; Zhu, G.Q.; Zhu, J.X.; Liang, X.L.; Zhu, Y.P.; He, H.P. Mechanisms for the Enhanced Photo-Fenton Activity of Ferrihydrite Modified with BiVO4 at Neutral pH. Appl. Catal. B: Environ. 2017, 212, 50–58. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Zhu, R.L.; Yan, L.X.; Fu, H.Y.; Xi, Y.F.; Zhou, H.J.; Zhu, G.Q.; He, H.P. Visible-Light Ag/AgBr/Ferrihydrite Catalyst with Enhanced Heterogeneous Photo-Fenton Reactivity via Electron Transfer From Ag/AgBr to Ferrihydrite. Appl. Catal. B: Environ. 2018, 239, 280–289. [Google Scholar] [CrossRef]
- Qian, X.F.; Wu, Y.W.; Kan, M.; Fang, M.Y.; Yue, D.T.; Zeng, J.; Zhao, Y.X. FeOOH Quantum Dots Coupled g-C3N4 for Visible Light Driving Photo-Fenton Degradation of Organic Pollutants. Appl. Catal. B: Environ. 2018, 237, 513–520. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Wang, L.Z.; Lei, J.Y.; Liu, Y.D.; Zhang, J.L. Photo-Fenton Degradation of Phenol by CdS/rGO/Fe2+ at Natural pH with In Situ-generated H2O2. Appl. Catal. B: Environ. 2019, 241, 367–374. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.K. Enhanced Formation of Sulfate Radicals by Metal-Doped BiFeO3 under Visible Light for Improving Photo-Fenton Catalytic Degradation of 2-Chlorophenol. Chem. Eng. J. 2017, 313, 1258–1268. [Google Scholar] [CrossRef]
- Hu, S.P.; Xu, C.Y.; Zhen, L. Solvothermal Synthesis of Bi2WO6 Hollow Structures with Excellent Visible-Light Photocatalytic Properties. Mater. Lett. 2013, 95, 117–120. [Google Scholar] [CrossRef]
- Sheng, J.Y.; Li, X.J.; Xu, Y.M. Generation of H2O2 and OH Radicals on Bi2WO6 for Phenol Degradation under Visible Light. ACS Catal. 2014, 4, 732–737. [Google Scholar] [CrossRef]
- Wang, D.J.; Guo, L.; Zhen, Y.Z.; Yue, L.L.; Xue, G.L.; Fu, F. AgBr Quantum Dots Decorated Mesoporous Bi2WO6 Architectures with Enhanced Photocatalytic Activities for Methylene Blue. J. Mater. Chem. A 2014, 2, 11716–11727. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, W.Z.; Wang, L.; Sun, S.M. Enhancement of Visible-Light Photocatalysis by Coupling with Narrow-Band-Gap Semiconductor: A Case Study on Bi2S3/Bi2WO6. ACS Appl. Mater. Interfaces 2012, 4, 593–597. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.Z.; Shang, M.; Sun, S.M.; Xu, J.H. Bi2WO6@Carbon/Fe3O4 Microspheres: Preparation, Growth Mechanism and Application in Water Treatment. J. Hazard. Mater. 2009, 172, 1193–1197. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, J.; Xiao, C.; Tan, X.K. Photocatalytic Degradation of Methylene Blue over Co3O4/Bi2WO6 Composite under Visible Light Irradiation. Catal. Commun. 2008, 9, 1247–1253. [Google Scholar] [CrossRef]
- Shan, G.Q.; Fu, Y.; Chu, X.L.; Chang, C.; Zhu, L.Y. Highly Active Magnetic Bismuth Tungstate/Magnetite Composite under Visible Light Irradiation in the Presence of Hydrogen Peroxide. J. Colloid Interface Sci. 2015, 444, 123–131. [Google Scholar] [CrossRef]
- Gao, L.G.; Du, J.W.; Ma, T.L. Cysteine-Assisted Synthesis of CuS-TiO2 Composites with Enhanced Photocatalytic Activity. Ceram. Int. 2017, 43, 9559–9563. [Google Scholar] [CrossRef]
- Cai, Z.L.; Zhou, Y.M.; Ma, S.S.; Li, S.W.; Yang, H.Y.; Zhao, S.; Zhong, X.; Wu, W.T. Enhanced Visible Light Photocatalytic Performance of g-C3N4/CuS p-n Heterojunctions for Degradation of Organic Dyes. J. Photoch. Photobio. A 2017, 348, 168–178. [Google Scholar] [CrossRef]
- Bhoi, Y.P.; Mishra, B.G. Photocatalytic Degradation of Alachlor using Type-II CuS/BiFeO3 Heterojunctions as Novel Photocatalyst under Visible Light Irradiation. Chem. Eng. J. 2018, 344, 391–401. [Google Scholar] [CrossRef]
- Bhoi, Y.P.; Behera, C.; Majhi, D.; Equeenuddin, S.M.; Mishra, B.G. Visible Light-Assisted Photocatalytic Mineralization of Diuron Pesticide using Novel type II CuS/Bi2W2O9 Heterojunctions with a Hierarchical Microspherical Structure. New J. Chem. 2018, 42, 281–292. [Google Scholar] [CrossRef]
- Zhao, W.; Liang, C.; Wang, B.B.; Xing, S.T. Enhanced Photocatalytic and Fenton-Like Performance of CuOx-Decorated ZnFe2O4. ACS Appl. Mater. Interfaces 2017, 9, 41927–41936. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Zhang, Y.F.; Lin, M.S.; Long, J.L.; Zhang, Z.Z.; Lin, H.X.; Wu, J.C.S.; Wang, X.X. Monolayered Bi2WO6 Nanosheets Mimicking Heterojunction Interface with Open Surfaces for Photocatalysis. Nat. Commun. 2015, 6, 8340. [Google Scholar] [CrossRef]
- Qian, X.F.; Yue, D.T.; Tian, Z.Y.; Ren, M.; Zhu, Y.; Kan, M.; Zhang, T.Y.; Zhao, Y.X. Carbon Quantum Dots Decorated Bi2WO6 Nanocomposite with Enhanced Photocatalytic Oxidation Activity for VOCs. Appl. Catal. B: Environ. 2016, 193, 16–21. [Google Scholar] [CrossRef]
- Wang, J.J.; Tang, L.; Zeng, G.M.; Deng, Y.C.; Dong, H.R.; Liu, Y.N.; Wang, L.L.; Peng, B.; Zhang, C.; Chen, F. 0D/2D Interface Engineering of Carbon Quantum Dots Modified Bi2WO6 Ultrathin Nanosheets with Enhanced Photoactivity for Full Spectrum Light Utilization and Mechanism Insight. Appl. Catal. B: Environ. 2018, 222, 115–123. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Jiang, W.J.; Luo, W.J.; Chen, X.J.; Zhu, Y.F. Ultrathin Nanosheets g-C3N4@Bi2WO6 Core-shell Structure via Low Temperature Reassembled Strategy to Promote Photocatalytic Activity. Appl. Catal. B: Environ. 2018, 237, 633–640. [Google Scholar] [CrossRef]
- Chen, S.B.; Lin, X.; Zhou, W.Y.; Zhang, S.S.; Fang, Y.P. Carbon-Coated Cu-TiO2 Nanocomposite with Enhanced Photostability and Photocatalytic Activity. Appl. Surf. Sci. 2019, 466, 254–261. [Google Scholar] [CrossRef]
- Wang, X.T.; Li, Y.; Zhang, X.Q.; Li, J.F.; Luo, Y.N.; Wang, C.W. Fabrication of a Magnetically Separable Cu2ZnSnS4/ZnFe2O4 p-n Heterostructured Nano-Photocatalyst for Synergistic Enhancement of Photocatalytic Activity Combining with Photo-Fenton Reaction. Appl. Surf. Sci. 2019, 479, 86–95. [Google Scholar] [CrossRef]
- Ruan, X.W.; Hu, H.; Che, H.N.; Jiang, E.H.; Zhang, X.X.; Liu, C.B.; Che, G.B. A Visible-Light-Driven Z-Scheme CdS/Bi12GeO20 Heterostructure with Enhanced Photocatalytic Degradation of Various Organics and the Reduction of Aqueous Cr(VI). J. Colloid Interface Sci. 2019, 543, 317–327. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.H.; Chen, Y.M.; Dai, J.; Sun, S.H. Enhanced Photocatalytic Activities of Visible-Light Driven Green Synthesis in Water and Environmental Remediation on Au/Bi2WO6 Hybrid Nanostructures. RSC Adv. 2015, 5, 9771–9782. [Google Scholar] [CrossRef]
- Das, K.; Majhi, D.; Bhoi, Y.P.; Mishra, B.G. Combustion Synthesis, Characterization and Photocatalytic Application of CuS/Bi4Ti3O12 p-n Heterojunction Materials towards Efficient Degradation of 2-Methyl-4-Chlorophenoxyacetic Acid Herbicide under Visible Light. Chem. Eng. J. 2019, 362, 588–599. [Google Scholar] [CrossRef]
- Yu, X.D.; Lin, X.C.; Feng, W.; Li, W.G. Effective Removal of Tetracycline by Using Bio-Templated Synthesis of TiO2/Fe3O4 Heterojunctions as a UV-Fenton Catalyst. Catal. Lett. 2019, 149, 552–560. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Zhang, G.K.; Li, J.; Wu, X.Y. 0D Bi Nanodots/2D Bi3NbO7 Nanosheets Heterojunctions for Efficient Visible Light Photocatalytic Degradation of Antibiotics: Enhanced Molecular Oxygen Activation and Mechanism Insight. Appl. Catal. B: Environ. 2019, 240, 39–49. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Q.; Zhong, J.B.; Huang, S.T.; Li, J.Z.; Song, J.B.; Burda, C. Enhanced Photocatalytic Performance of Ag2O/BiOF Composite Photocatalysts Originating from Efficient Interfacial Charge Separation. Appl. Surf. Sci. 2017, 416, 666–671. [Google Scholar] [CrossRef]
- Ma, D.M.; Zhong, J.B.; Li, J.Z.; Burda, C.; Duan, R. Preparation and Photocatalytic Performance of MWCNTs/BiOCl: Evidence for the Superoxide Radical Participation in the Degradation Mechanism of phenol. Appl. Surf. Sci. 2019, 480, 395–403. [Google Scholar] [CrossRef]
- Yang, J.; Dai, J.; Chen, C.C.; Zhao, J.C. Effects of Hydroxyl Radicals and Oxygen Species on the 4-Chlorophenol Degradation by Photoelectrocatalytic Reactions with TiO2-Film Electrodes. J. Photochem. Photobio. A: Chem. 2009, 208, 66–77. [Google Scholar] [CrossRef]
- Cai, T.; Liu, Y.T.; Wang, L.L.; Dong, W.Y.; Chen, H.; Zeng, W.G.; Xia, X.N.; Zeng, G.M. Activation of Persulfate by Photoexcited Dye for Antibiotic Degradation: Radical and Nonradical Reactions. Chem. Eng. J. [CrossRef]
- Liu, L.D.; Wang, Y.; Liu, Q.; Wang, W.J.; Duan, L.; Yang, X.; Yi, S.X.; Xue, X.T.; Zhang, J.W. Activation Peroxydisulfate by Morphology-Dependent NiO Catalysts: Structural Origin of Different Catalytic Properties. Appl. Catal. B: Environ. [CrossRef]
- Fu, F.; Shen, H.D.; Sun, X.; Xue, W.W.; Shoneyec, A.; Ma, J.N.; Luo, L.; Wang, D.J.; Wang, J.G.; Tang, J.W. Synergistic Effect of Surface Oxygen Vacancies and Interfacial Charge Transfer on Fe(III)/Bi2MoO6 for Efficient Photocatalysis. Appl. Catal. B: Environ. 2019, 247, 150–162. [Google Scholar] [CrossRef]














| Samples | Light Source | Organic Pollutants | Experimental Conditions | ηd) (%) | Refs. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type a) | Power (W) | Type | Conc. b) (mg·L−1) | Catalyst (g/L) | t c) (time) | pH | H2O2 | |||
| Co3O4/Bi2WO6 | Tungsten lamp | 100 | Methylene blue (MB) | 10 | 1 | 80 | 8 | 1.96 mM | 96 | [26] |
| Bi2WO6/Fe3O4 | Xe lamp | 350 | RhB | 10 | 0.5 | 120 | 7 | 10 mM | 95 | [27] |
| CuS-TiO2 | Xe lamp | 350 | MB | 10 | 1 | 180 | - | - | 100 | [28] |
| g-C3N4/CuS | Xe lamp | 300 | RhB | 10 | 0.3 | 60 | - | - | 96.8 | [29] |
| CuS/Bi2W2O9 | Xe lamp | 150 | Diuron | 10 | 0.75 | 180 | - | 200 µL | 95 | [31] |
| CuS/Bi4Ti3O12 | Xe lamp | 250 | 2-methyl-4-chlorophenoxyacetic acid | 10 | 0.25 | 180 | - | 150 µL | 96 | [41] |
| TiO2/Fe3O4 | UVC-lamp | 10 | TC-HCl | 50 | 0.3 | 60 | 7 | 10 mM | 98 | [42] |
| 0.1% CuS/Bi2WO6 | Metal halide lamp | 300 | RhB | 20 | 1.0 | 25 | - | 100 µL | 100 | this work |
| TC-HCl | 40 | 1.0 | 50 | 7 | 73 | |||||
| Samples | τ1 (ns) | A1 | τ2 (ns) | A2 | Τav (ns) |
|---|---|---|---|---|---|
| Bi2WO6 nanosheets | 0.903 | 2.22 × 1011 | 12.205 | 1022.997 | 0.903 |
| 0.1% CuS/Bi2WO6 | 17.552 | 1.50 × 103 | 1.359 | 1.32 × 109 | 1.360 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Zhang, K.; Han, X.; Zhao, Q.; Wang, D.; Fu, F. 2D In-Plane CuS/Bi2WO6 p-n Heterostructures with Promoted Visible-Light-Driven Photo-Fenton Degradation Performance. Nanomaterials 2019, 9, 1151. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9081151
Guo L, Zhang K, Han X, Zhao Q, Wang D, Fu F. 2D In-Plane CuS/Bi2WO6 p-n Heterostructures with Promoted Visible-Light-Driven Photo-Fenton Degradation Performance. Nanomaterials. 2019; 9(8):1151. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9081151
Chicago/Turabian StyleGuo, Li, Kailai Zhang, Xuanxuan Han, Qiang Zhao, Danjun Wang, and Feng Fu. 2019. "2D In-Plane CuS/Bi2WO6 p-n Heterostructures with Promoted Visible-Light-Driven Photo-Fenton Degradation Performance" Nanomaterials 9, no. 8: 1151. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9081151