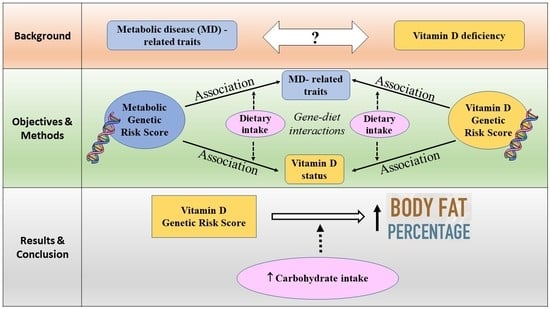
Interaction between Vitamin D-Related Genetic Risk Score and Carbohydrate Intake on Body Fat Composition: A Study in Southeast Asian Minangkabau Women
Abstract
:1. Introduction
2. Methodology
2.1. Study Population
2.2. Anthropometric Measures
2.3. Biochemical Measures
2.4. Assessment of Dietary Intake
2.5. SNP Selection and Genotyping
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Association between Metabolic-GRS and Anthropometric and Biochemical Measurements
3.3. Association between Vitamin D-GRS and Anthropometric and Biochemical Measurements
3.4. Interaction between the Vitamin D-GRS and Dietary Factors on Biochemical and Anthropometric Parameters
3.5. Interaction between the Metabolic-GRS and Dietary Factors on Clinical and Biochemical Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2D | type 2 diabetes |
| WC | waist circumference |
| BMI | body mass index |
| BFP | body fat percentage |
| HbA1c | glycated haemoglobin |
| LDL-c | low density lipoprotein cholesterol |
| HDL-c | high density lipoprotein cholesterol |
| DHCR7 | 7-dehydrocholesterol reductase |
| CYP2R1 | 25-hydroxylase |
| CYP24A1 | 24-hydroxylase |
| DBP | vitamin D binding protein |
| GC | group-specific component |
| CASR | calcium sensing receptor |
| FTO | fat mass and obesity-associated gene |
| TCF7L2 | transcription factor 7-like 2 gene |
| MC4R | melanocortin 4 receptor gene |
| KCNQ1 | potassium voltage-gated channel subfamily Q member 1 |
| CDKN2A/B | cyclin dependent kinase inhibitor 2A/B |
| GRS | genetic risk score |
| SNP | single nucleotide polymorphism |
| MR | Mendelian Randomization |
| GWAS | genome wide association studies |
| MINANG | Minangkabau Indonesia study on nutrition and genetics |
| WHO | World Health Organization |
| FFQ | food frequency questionnaire |
| HWE | Hardy Weinberg equilibrium |
| SD | standard deviation |
| ELISA | enzyme-linked immunosorbent assay |
| SQ-FFQ | semi-quantitative food frequency questionnaire |
References
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Alathari, B.E.; Sabta, A.A.; Kalpana, C.A.; Vimaleswaran, K.S. Vitamin D pathway-related gene polymorphisms and their association with metabolic diseases: A literature review. J. Diabetes Metab. Disord. 2020. [Google Scholar] [CrossRef]
- Jiang, X.; Kiel, D.P.; Kraft, P. The genetics of vitamin D. Bone 2019, 126, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Been, L.F.; Ralhan, S.; Wander, G.S.; Mehra, N.K.; Singh, J.; Mulvihill, J.J.; Aston, C.E.; Sanghera, D.K. Variants in KCNQ1 increase type II diabetes susceptibility in South Asians: A study of 3310 subjects from India and the US. BMC Med. Genet. 2011, 12, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bego, T.; Čaušević, A.; Dujić, T.; Malenica, M.; Velija-Asimi, Z.; Prnjavorac, B.; Marc, J.; Nekvindová, J.; Palička, V.; Semiz, S. Association of FTO Gene Variant (rs8050136) with Type 2 Diabetes and Markers of Obesity, Glycaemic Control and Inflammation. J. Med. Biochem. 2019, 38, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Chidambaram, M.; Liju, S.; Saboo, B.; Sathyavani, K.; Viswanathan, V.; Pankratz, N.; Gross, M.; Mohan, V.; Radha, V. Replication of genome-wide association signals in Asian Indians with early-onset type 2 diabetes. Acta Diabetol. 2016, 53, 915–923. [Google Scholar] [CrossRef]
- Chris, A.; Narila, N.; Flori, S.; Risfy, P.; Intan, A.; Zeti, H.; Witri, A.; Hari, H.; Nouval, S.; Endah, W.; et al. Preliminary study: Identification of dna variation with snp numbers rs1137101 and rs8050136 in patient’s type 2 diabetes mellitus at salsabila clinic bogor—Indonesia. Asian J. Microbiol. Biotechnol. Environ. Sci. 2019, 21, 931–934. [Google Scholar]
- Gupta, V.; Khadgawat, R.; Ng, H.K.T.; Walia, G.K.; Kalla, L.; Rao, V.R.; Sachdeva, M.P. Association of TCF7L2 and ADIPOQ with body mass index, waist-hip ratio, and systolic blood pressure in an endogamous ethnic group of India. Genet. Test. Mol. Biomark. 2012, 16, 948–951. [Google Scholar] [CrossRef] [Green Version]
- Prakash, J.; Srivastava, N.; Awasthi, S.; Agarwal, C.; Natu, S.; Rajpal, N.; Mittal, B. Association of PPAR-γ gene polymorphisms with obesity and obesity-associated phenotypes in north indian population. Am. J. Hum. Biol. 2012, 24, 454–459. [Google Scholar] [CrossRef]
- Srivastava, A.; Mittal, B.; Prakash, J.; Narain, V.S.; Natu, S.M.; Srivastava, N. Evaluation of MC4R [rs17782313, rs17700633], AGRP [rs3412352] and POMC [rs1042571] Polymorphisms with Obesity in Northern India. Oman Med. J. 2014, 29, 114–118. [Google Scholar] [CrossRef]
- Chauhan, G.; Spurgeon, C.J.; Tabassum, R.; Bhaskar, S.; Kulkarni, S.R.; Mahajan, A.; Chavali, S.; Kumar, M.V.K.; Prakash, S.; Dwivedi, O.P.; et al. Impact of common variants of PPARG, KCNJ11, TCF7L2, SLC30A8, HHEX, CDKN2A, IGF2BP2, and CDKAL1 on the risk of type 2 diabetes in 5,164 Indians. Diabetes 2010, 59, 2068–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vimaleswaran, K.S.; Loos, R.J. Progress in the genetics of common obesity and type 2 diabetes. Expert Rev. Mol. Med. 2010, 12, e7. [Google Scholar] [CrossRef] [PubMed]
- Parrillo, L.; Spinelli, R.; Nicolò, A.; Longo, M.; Mirra, P.; Raciti, G.A.; Miele, C.; Beguinot, F. Nutritional Factors, DNA Methylation, and Risk of Type 2 Diabetes and Obesity: Perspectives and Challenges. Int. J. Mol. Sci. 2019, 20, 2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angkurawaranon, C.; Jiraporncharoen, W.; Chenthanakij, B.; Doyle, P.; Nitsch, D. Urban environments and obesity in southeast Asia: A systematic review, meta-analysis and meta-regression. PLoS ONE 2014, 9, e113547. [Google Scholar]
- Aji, A.S.; Erwinda, E.; Rasyid, R.; Yusrawati, Y.; Malik, S.G.; Alathari, B.; Lovegrove, J.A.; Lipoeto, N.I.; Vimaleswaran, K.S. A genetic approach to study the relationship between maternal Vitamin D status and newborn anthropometry measurements: The Vitamin D pregnant mother (VDPM) cohort study. J. Diabetes Metab. Disord. 2020, 19, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Fajarini, I.A.; Sartika, R.A.D. Obesity as Type 2 Diabetes Common Comorbidity: Study of Type 2 Diabetes Patients’ Eating Behaviour and Other Determinants in Jakarta, Indonesia. Kesmas Natl. Public Health J. 2019, 13, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Harbuwono, D.S.; Pramono, L.A.; Yunir, E.; Subekti, I. Obesity and central obesity in Indonesia: Evidence from a national health survey. Med. J. Indones 2018, 27, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Arifin, B.; van Asselt, A.D.I.; Setiawan, D.; Atthobari, J.; Postma, M.J.; Cao, Q. Diabetes distress in Indonesian patients with type 2 diabetes: A comparison between primary and tertiary care. BMC Health Serv. Res. 2019, 19, 773. [Google Scholar] [CrossRef]
- Ligita, T.; Wicking, K.; Francis, K.; Harvey, N.; Nurjannah, I. How people living with diabetes in Indonesia learn about their disease: A grounded theory study. PLoS ONE 2019, 14, e0212019. [Google Scholar]
- Surendran, S.; Aji, A.S.; Ariyasra, U.; Sari, S.R.; Malik, S.G.; Tasrif, N.; Yani, F.F.; Lovegrove, J.A.; Sudji, I.R.; Lipoeto, N.I.; et al. A nutrigenetic approach for investigating the relationship between vitamin B12 status and metabolic traits in Indonesian women. J. Diabetes Metab. Disord. 2019, 18, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, A. The Matrilineal System of the Minangkabau and its Persistence Throughout History: A Structural Perspective. Southeast Asia Multidiscip. J. 2013, 13, 1–13. [Google Scholar]
- Dudbridge, F. Polygenic Epidemiology. Genet. Epidemiol. 2016, 40, 268–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hüls, A.; Krämer, U.; Carlsten, C.; Schikowski, T.; Ickstadt, K.; Schwender, H. Comparison of weighting approaches for genetic risk scores in gene-environment interaction studies. BMC Genet. 2017, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S. A nutrigenetics approach to study the impact of genetic and lifestyle factors on cardiometabolic traits in various ethnic groups: Findings from the GeNuIne Collaboration. Proc. Nutr. Soc. 2020, 79, 194–204. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S. Gene–nutrient interactions on metabolic diseases: Findings from the GeNuIne Collaboration. Nutr. Bull. 2017, 42, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.H.; Yeh, W.T. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: An extension of Asian-Pacific recommendations. Asia Pac. J. Clin. Nutr. 2008, 17, 370–374. [Google Scholar]
- Lipoeto, N.I.; Agus, Z.; Oenzil, F.; Wahlqvist, M.; Wattanapenpaiboon, N. Dietary intake and the risk of coronary heart disease among the coconut-consuming Minangkabau in West Sumatra, Indonesia. Asia Pac. J. Clin. Nutr. 2004, 13, 377–384. [Google Scholar]
- Elkum, N.; Alkayal, F.; Noronha, F.; Ali, M.M.; Melhem, M.; Al-Arouj, M.; Bennakhi, A.; Behbehani, K.; Alsmadi, O.; Abubaker, J. Vitamin D insufficiency in Arabs and South Asians positively associates with polymorphisms in GC and CYP2R1 genes. PLoS ONE 2014, 9, e113102. [Google Scholar] [CrossRef]
- Foucan, L.; Vélayoudom-Céphise, F.-L.; Larifla, L.; Armand, C.; Deloumeaux, J.; Fagour, C.; Plumasseau, J.; Portlis, M.-L.; Liu, L.; Bonnet, F.; et al. Polymorphisms in GC and NADSYN1 Genes are associated with vitamin D status and metabolic profile in Non-diabetic adults. BMC Endocr. Disord. 2013, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Grzegorzewska, A.E.; Bednarski, D.; Świderska, M.; Mostowska, A.; Jagodziński, P.P. The Calcium-Sensing Receptor Gene Polymorphism rs1801725 and Calcium-Related Phenotypes in Hemodialysis Patients. Kidney Blood Press. Res. 2018, 43, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Grzegorzewska, A.E.; Frycz, B.A.; Świderska, M.; Niepolski, L.; Mostowska, A.; Jagodziński, P.P. Calcium-sensing receptor gene (CASR) polymorphisms and CASR transcript level concerning dyslipidemia in hemodialysis patients: A cross-sectional study. BMC Nephrol. 2019, 20, 436. [Google Scholar]
- Kwak, S.Y.; Yongjoo Park, C.; Jo, G.; Yoen Kim, O.; Shin, M.J. Association among genetic variants in the vitamin D pathway and circulating 25-hydroxyvitamin D levels in Korean adults: Results from the Korea National Health and Nutrition Examination Survey 2011–2012. Endocr. J. 2018, 65, 881–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rooney, M.R.; Pankow, J.S.; Sibley, S.D.; Selvin, E.; Reis, J.P.; Michos, E.D.; Lutsey, P.L. Serum calcium and incident type 2 diabetes: The Atherosclerosis Risk in Communities (ARIC) study. Am. J. Clin. Nutr. 2016, 104, 1023–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Mao, J.; Zhang, M.; Liu, H.; Li, H.; Lei, H.; Han, L.; Gao, M. Vitamin D Deficiency in Uygurs and Kazaks Is Associated with Polymorphisms in CYP2R1 and DHCR7/NADSYN1 Genes. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1960–1968. [Google Scholar]
- Zhang, Y.; Wang, X.; Liu, Y.; Qu, H.; Qu, S.; Wang, W.; Ren, L. The GC, CYP2R1 and DHCR7 genes are associated with vitamin D levels in northeastern Han Chinese children. Swiss Med. Wkly. 2012, 142, w13636. [Google Scholar] [CrossRef] [PubMed]
- Alathari, B.E.; Bodhini, D.; Jayashri, R.; Lakshmipriya, N.; Shanthi Rani, C.S.; Sudha, V.; Lovegrove, J.A.; Anjana, R.M.; Mohan, V.; Radha, V.; et al. A Nutrigenetic Approach to Investigate the Relationship between Metabolic Traits and Vitamin D Status in an Asian Indian Population. Nutrients 2020, 12, 1357. [Google Scholar] [CrossRef]
- Apalasamy, Y.D.; Ming, M.F.; Rampal, S.; Bulgiba, A.; Mohamed, Z. Association of melanocortin-4 receptor gene polymorphisms with obesity-related parameters in Malaysian Malays. Ann. Hum. Biol. 2013, 40, 102–106. [Google Scholar] [CrossRef]
- Chu, A.Y.; Workalemahu, T.; Paynter, N.P.; Rose, L.M.; Giulianini, F.; Tanaka, T.; Ngwa, J.S.; Qi, Q.; Curhan, G.C.; Rimm, E.B.; et al. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum. Mol. Genet. 2013, 22, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- Vasan, S.K.; Karpe, F.; Gu, H.F.; Brismar, K.; Fall, C.H.; Ingelsson, E.; Fall, T. FTO genetic variants and risk of obesity and type 2 diabetes: A meta-analysis of 28,394 Indians. Obesity 2014, 22, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xiao, J.; He, J.; Zhang, X.; Hong, J.; Kong, X.; Mills, K.T.; Weng, J.; Jia, W.; Yang, W. Cross-Sectional and Longitudinal Replication Analyses of Genome-Wide Association Loci of Type 2 Diabetes in Han Chinese. PLoS ONE 2014, 9, e91790. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal relationship between obesity and vitamin D status: Bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Ahn, J.; Yu, K.; Stolzenberg-Solomon, R.; Simon, K.C.; McCullough, M.L.; Gallicchio, L.; Jacobs, E.J.; Ascherio, A.; Helzlsouer, K.; Jacobs, K.B.; et al. Genome-wide association study of circulating vitamin D levels. Hum. Mol. Genet. 2010, 19, 2739–2745. [Google Scholar] [CrossRef]
- Hiraki, L.T.; Major, J.M.; Chen, C.; Cornelis, M.C.; Hunter, D.J.; Rimm, E.B.; Simon, K.C.; Weinstein, S.J.; Purdue, M.P.; Yu, K.; et al. Exploring the Genetic Architecture of Circulating 25-Hydroxyvitamin, D. Genet. Epidemiol. 2013, 37, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; O’Reilly, P.F.; Aschard, H.; Hsu, Y.H.; Richards, J.B.; Dupuis, J.; Ingelsson, E.; Karasik, D.; Pilz, S.; Berry, D.; et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018, 9, 260. [Google Scholar] [CrossRef]
- Sapkota, B.R.; Hopkins, R.; Bjonnes, A.; Ralhan, S.; Wander, G.S.; Mehra, N.K.; Singh, J.R.; Blackett, P.R.; Saxena, R.; Sanghera, D.K. Genome-wide association study of 25(OH) Vitamin D concentrations in Punjabi Sikhs: Results of the Asian Indian diabetic heart study. J. Steroid Biochem. Mol. Biol. 2016, 158, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Whittaker, J.C.; Power, C.; Jarvelin, M.R.; Hypponen, E. Genetic association analysis of vitamin D pathway with obesity traits. Int. J. Obes. (Lond.) 2013, 37, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
- Narvaez, C.J.; Matthews, D.; Broun, E.; Chan, M.; Welsh, J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology 2009, 150, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.E.; Kong, J.; Zhang, W.; Szeto, F.L.; Ye, H.; Deb, D.K.; Brady, M.J.; Li, Y.C. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J. Biol. Chem. 2011, 286, 33804–33810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usfar, A.A.; Fahmida, U. Do Indonesians follow its Dietary Guidelines?: Evidence related to food consumption, healthy lifestyle, and nutritional status within the period 2000–2010. Asia Pac. J. Clin. Nutr. 2011, 20, 484–494. [Google Scholar] [PubMed]
- Ministry of Health Republic of Indonesia. The Indonesian Dietary Recommendation (AKG—Angka Kecukupan Gizi); Ministry of Health Republic of Indonesia: Jakarta, Indonesia, 2019.
- Sartorius, K.; Sartorius, B.; Madiba, T.E.; Stefan, C. Does high-carbohydrate intake lead to increased risk of obesity? A systematic review and meta-analysis. BMJ Open 2018, 8, e018449. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.L.; Esteves, S.S.; da Costa Pereira, A.; Yancy, W.S., Jr.; Nunes, J.P. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes. Rev. 2012, 13, 1048–1066. [Google Scholar] [CrossRef]
- Unwin, D.J.; Tobin, S.D.; Murray, S.W.; Delon, C.; Brady, A.J. Substantial and Sustained Improvements in Blood Pressure, Weight and Lipid Profiles from a Carbohydrate Restricted Diet: An Observational Study of Insulin Resistant Patients in Primary Care. Int. J. Environ. Res. Public Health 2019, 16, 2680. [Google Scholar] [CrossRef] [Green Version]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef]
- Newton, A.L.; Hanks, L.J.; Ashraf, A.P.; Williams, E.; Davis, M.; Casazza, K. Macronutrient intake influences the effect of 25-hydroxy-vitamin d status on metabolic syndrome outcomes in african american girls. Cholesterol 2012, 2012, 581432. [Google Scholar] [CrossRef] [Green Version]
- Linnemann, A.K.; Baan, M.; Davis, D.B. Pancreatic β-cell proliferation in obesity. Adv. Nutr. 2014, 5, 278–288. [Google Scholar] [CrossRef] [Green Version]
- Lipoeto, N.I.; Agus, Z.; Oenzil, F.; Masrul, M.; Wattanapenpaiboon, N.; Wahlqvist, M.L. Contemporary Minangkabau food culture in West Sumatra, Indonesia. Asia Pac. J. Clin. Nutr. 2001, 10, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Adab, P.; Pallan, M.; Whincup, P.H. Is BMI the best measure of obesity? BMJ 2018, 360, k1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, C.L.; Yan, E.; Chen, S.; Hong, K.; Arechiga, A.; Kim, W.S.; Deng, M.; Li, Z.; Heber, D. Body fat and body-mass index among a multiethnic sample of college-age men and women. J. Obes. 2013, 2013, 790654. [Google Scholar] [CrossRef] [PubMed]
- Alsulami, S.; Aji, A.S.; Ariyasra, U.; Sari, S.R.; Tasrif, N.; Yani, F.F.; Lovegrove, J.A.; Sudji, I.R.; Lipoeto, N.I.; Vimaleswaran, K.S. Interaction between the genetic risk score and dietary protein intake on cardiometabolic traits in Southeast Asian. Genes Nutr. 2020, 15, 19. [Google Scholar] [CrossRef] [PubMed]



| Characteristics of Study Participants | Vitamin D Sufficiency (≥20 ng/mL) (n = 44) | Vitamin D Insufficiency (≥12 ≤ 19 ng/mL) (n = 44) | Vitamin D Deficiency <12 ng/mL (n = 22) | p Value |
|---|---|---|---|---|
| Age (years) | 43.8 ± 7.8 | 40.0 ± 10.9 | 34.3 ± 10.3 | 0.001 |
| BMI (kg/m2) | 25.7 ± 4.4 | 25.3 ± 4.0 | 24.1 ± 4.3 | 0.358 |
| WC (cm) | 86.5 ± 9.9 | 82.6 ± 11.3 | 81.0 ± 8.6 | 0.070 |
| BFP (%) | 36.2 ± 7.8 | 36.4 ± 6.5 | 34.0 ± 6.3 | 0.392 |
| Glucose (mg/dL) | 96.1 ± 30.4 | 92.1 ± 9.0 | 86.4 ± 10.6 | 0.198 |
| HbA1c (ng/mL) | 710 ± 703 | 670 ± 573 | 440 ± 209 | 0.178 |
| Fasting Insulin (nmlU/L) | 36,020 ± 29,916 | 32,915 ± 24,107 | 22,261 ± 8092 | 0.101 |
| Total Cholesterol (mg/dL) | 209 ± 46 | 216 ± 44 | 194 ± 37 | 0.142 |
| HDL Cholesterol (mg/dL) | 57.4 ± 8.4 | 59.9 ± 11.4 | 61.2 ± 11.4 | 0.295 |
| LDL Cholesterol (mg/dL) | 127.3 ± 40.3 | 135.4 ± 41.9 | 113.8 ± 31.8 | 0.115 |
| Serum Triglycerides (mg/dL) | 103 ± 44.9 | 93.8 ± 41.7 | 96.7 ± 44.0 | 0.601 |
| Total Energy Intake (kcal) | 1797.0 ± 645.0 | 1695.0 ± 545.0 | 1894.3 ± 675.4 | 0.442 |
| Carbohydrate (g) | 233.7 ± 75.1 | 225.9 ± 58.9 | 246.0 ± 86.5 | 0.563 |
| Protein (g) | 77.2 ± 41.7 | 74.2 ± 30.4 | 82.2 ± 39.2 | 0.707 |
| Fat (g) | 61.2 ± 36.1 | 54.7 ± 31.6 | 64.1 ± 32.1 | 0.498 |
| Dietary Fibre (g) | 8.6 ± 4.3 | 8.3 ± 3.5 | 10.2 ± 5.5 | 0.218 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alathari, B.E.; Aji, A.S.; Ariyasra, U.; Sari, S.R.; Tasrif, N.; Yani, F.F.; Sudji, I.R.; Lovegrove, J.A.; Lipoeto, N.I.; Vimaleswaran, K.S. Interaction between Vitamin D-Related Genetic Risk Score and Carbohydrate Intake on Body Fat Composition: A Study in Southeast Asian Minangkabau Women. Nutrients 2021, 13, 326. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020326
Alathari BE, Aji AS, Ariyasra U, Sari SR, Tasrif N, Yani FF, Sudji IR, Lovegrove JA, Lipoeto NI, Vimaleswaran KS. Interaction between Vitamin D-Related Genetic Risk Score and Carbohydrate Intake on Body Fat Composition: A Study in Southeast Asian Minangkabau Women. Nutrients. 2021; 13(2):326. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020326
Chicago/Turabian StyleAlathari, Buthaina E., Arif Sabta Aji, Utami Ariyasra, Sri R. Sari, Nabila Tasrif, Finny F. Yani, Ikhwan R. Sudji, Julie A. Lovegrove, Nur I. Lipoeto, and Karani S. Vimaleswaran. 2021. "Interaction between Vitamin D-Related Genetic Risk Score and Carbohydrate Intake on Body Fat Composition: A Study in Southeast Asian Minangkabau Women" Nutrients 13, no. 2: 326. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020326