Hypothalamic Renin–Angiotensin System and Lipid Metabolism: Effects of Virgin Olive Oil versus Butter in the Diet
Abstract
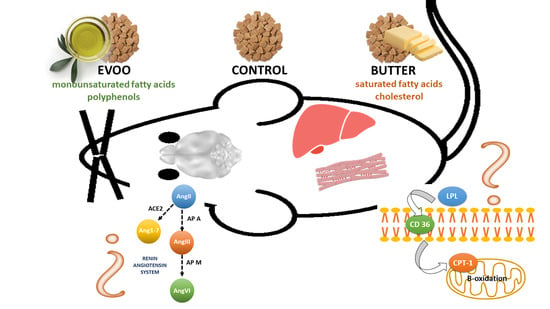
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Determination of Aminopeptidase A Activity
2.3. RNA Isolation and Real-Time PCR of Aminopeptidase A
2.4. Angiotensins UHPLC MS/MS
2.5. RNA Isolation and Real-Time PCR of Genes Involved in Lipid Metabolism
2.6. Statistical Analysis
3. Results
3.1. Effects of Diets on Body Weight and Total Visceral Adipose Tissue
3.2. Hypothalamic Angiotensin Profile
3.3. Hypothalamic Aminopeptidase A Activity and Expression
3.4. Hepatic Expression of Genes Involved in Lipid Metabolism
3.5. Skeletal Muscle Expression of Genes Involved in Lipid Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J.H. Dietary Monounsaturated Fatty Acids Are Protective Against Metabolic Syndrome and Cardiovascular Disease Risk Factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Julibert, A.; Bibiloni, M.D.M.; Bouzas, C.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Zomeño, M.D.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Total and Subtypes of Dietary Fat Intake and Its Association with Components of the Metabolic Syndrome in a Mediterranean Population at High Cardiovascular Risk. Nutrients 2019, 11, 1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Sánchez, M.; Prieto, I.; Wangensteen, R.; Banegas, I.; Segarra, A.B.; Villarejo, A.B.; Vives, F.; Cobo, J.; De Gasparo, M. The renin-angiotensin system: New insight into old therapies. Curr. Med. Chem. 2013, 20, 1313–1322. [Google Scholar] [CrossRef]
- Grobe, J.L.; Grobe, C.L.; Beltz, T.G.; Westphal, S.G.; Morgan, D.A.; Xu, D.; De Lange, W.J.; Li, H.; Sakai, K.; Thedens, D.R.; et al. The brain Renin–angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab. 2010, 12, 431–442. [Google Scholar] [CrossRef] [Green Version]
- De Kloet, A.D.; Krause, E.G.; Scott, K.A.; Foster, M.T.; Herman, J.P.; Sakai, R.R.; Seeley, R.J.; Woods, S.C. Central angiotensin II has catabolic action at white and brown adipose tissue. Am. J. Physiol. Metab. 2011, 301, E1081–E1091. [Google Scholar] [CrossRef] [Green Version]
- Claflin, K.E.; Grobe, J.L. Control of energy balance by the brain renin–angiotensin system. Curr. Hypertens. Rep. 2015, 17, 38. [Google Scholar] [CrossRef]
- Bruce, E.B.; De Kloet, A.D. The intricacies of the renin-angiotensin-system in metabolic regulation. Physiol. Behav. 2017, 178, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Prieto, I.; Segarra, A.B.; De Gasparo, M.; Martínez-Cañamero, M.; Ramírez-Sánchez, M. Divergent profile between hypothalamic and plasmatic aminopeptidase activities in WKY and SHR. Influence of beta-adrenergic blockade. Life Sci. 2018, 192, 9–17. [Google Scholar] [CrossRef]
- De Kloet, A.D.; Krause, E.G.; Woods, S.C. The renin angiotensin system and the metabolic syndrome. Physiol. Behav. 2010, 100, 525–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, G.; Grobe, J.L. The renin–angiotensin system in the arcuate nucleus controls resting metabolic rate. Curr. Opin. Nephrol. Hypertens. 2019, 28, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K. The Mouse Brain in Stereotaxic Coordinates, 4th ed.; Elsevier Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martínez-Rodríguez, A.M.; Cobo, A.; Ramírez, M.; Abriouel, H.; Gálvez, A.; Martínez-Cañamero, M. Influence of a diet enriched with virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS ONE 2018, 13, e0190368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, M.; Prieto, I.; Banegas, I.; Segarra, A.B.; Alba, F. Neuropeptidases. Methods Mol. Biol. 2011, 789, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Oike, H.; Ogawa, Y.; Azami, K. Long-Term Feeding of a High-Fat Diet Ameliorated Age-Related Phenotypes in SAMP8 Mice. Nutrients 2020, 12, 1416. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, N.; Gavino, V.; Receveur, O. Could the quality of dietary fat, and not just its quantity, be related to risk of obesity? Obesity 2008, 16, 7–15. [Google Scholar] [CrossRef]
- Krishnan, S.; Cooper, J.A. Effect of dietary fatty acid composition on substrate utilization and body weight maintenance in humans. Eur. J. Nutr. 2014, 53, 691–710. [Google Scholar] [CrossRef]
- Benítez-Arciniega, A.D.; Gómez-Ulloa, D.; Vilà, A.; Giralt, L.; Colprim, D.; Martori, M.-A.R.; Schröder, H. Olive oil consumption, BMI, and risk of obesity in Spanish adults. Obes. Facts 2012, 5, 52–59. [Google Scholar] [CrossRef]
- Domínguez-Vías, G.; Aretxaga-Maza, G.; Prieto, I.; Luna, J.D.D.; De Gasparo, M.; Ramírez-Sánchez, M. Diurnal opposite variation between angiotensinase activities in photo-neuro-endocrine tissues of rats. Chronobiol. Int. 2017, 34, 1180–1186. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, A.; Wang, Q.; Zhou, J.R. High Oleic Acid Peanut Oil and Extra Virgin Olive Oil Supplementation Attenuate Metabolic Syndrome in Rats by Modulating the Gut Microbiota. Nutrients 2019, 11, 3005. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Vías, G.; Segarra, A.B.; Ramírez-Sánchez, M.; Prieto, I. The Role of High Fat Diets and Liver Peptidase Activity in the Development of Obesity and Insulin Resistance in Wistar Rats. Nutrients 2020, 12, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claro, C.; Quintela, J.C.; Pérez-Montero, M.; Miñano, F.J.; De Sotomayor, M.A.; Herrera, M.D.; Rodríguez-Rodríguez, R. Pomace Olive Oil Concentrated in Triterpenic Acids Restores Vascular Function, Glucose Tolerance and Obesity Progression in Mice. Nutrients 2020, 12, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo, M.; Prieto, I.; Abriouel, H.; Villarejo, A.B.; Ramírez-Sánchez, M.; Cobo, A.; Benomar, N.; Gálvez, A.; Martínez-Cañamero, M. Changes in Gut Microbiota Linked to a Reduction in Systolic Blood Pressure in Spontaneously Hypertensive Rats Fed an Extra Virgin Olive Oil-Enriched Diet. Plant Foods Hum. Nutr. 2017, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Martínez, N.; Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martínez-Rodríguez, A.M.; Cobo, A.; Ramírez-Sánchez, M.; Galvez, A.; Martínez-Cañamero, M. Refined versus Extra Virgin Olive Oil High-Fat Diet Impact on Intestinal Microbiota of Mice and Its Relation to Different Physiological Variables. Microorganisms 2019, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Cano, P.; Cardinali, D.P.; Ríos-Lugo, M.J.; Fernández-Mateos, M.P.; Toso, C.F.R.; Esquifino, A.I. Effect of a high-fat diet on 24-hour pattern of circulating adipocytokines in rats. Obesity 2009, 17, 1866–1871. [Google Scholar] [CrossRef]
- Kentish, S.J.; Wittert, G.A.; Blackshaw, L.A.; Page, A.J. A chronic high fat diet alters the homologous and heterologous control of appetite regulating peptide receptor expression. Peptides 2013, 46, 150–158. [Google Scholar] [CrossRef]
- Shen, Y.; Song, S.J.; Keum, N.; Park, T. Olive leaf extract attenuates obesity in high-fat diet-fed mice by modulating the expression of molecules involved in adipogenesis and thermogenesis. Evid.-Based Complement. Altern. Med. 2014, 2014, 971890. [Google Scholar] [CrossRef] [Green Version]
- Jurado-Ruiz, E.; Varela, L.M.; Luque, A.; Berná, G.; Cahuana, G.; Martínez-Force, E.; Gallego-Durán, R.; Soria, B.; De Roos, B.; Gómez, M.R.; et al. An extra virgin olive oil rich diet intervention ameliorates the nonalcoholic steatohepatitis induced by a high-fat “Western-type” diet in mice. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Hilzendeger, A.M.; Morgan, N.A.; Brooks, L.; Dellsperger, D.; Liu, X.; Grobe, J.L.; Rahmouni, K.; Sigmund, C.D.; Mark, A.L. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am. J. Physiol. Circ. Physiol. 2012, 303, H197–H206. [Google Scholar] [CrossRef] [Green Version]
- Kasper, S.O.; Carter, C.S.; Ferrario, C.M.; Ganten, D.; Ferder, L.F.; Sonntag, W.E.; Gallagher, P.E.; Diz, D.I. Growth, metabolism, and blood pressure disturbances during aging in transgenic rats with altered brain renin-angiotensin systems. Physiol. Genom. 2005, 23, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Winkler, M.; Schuchard, J.; Stölting, I.; Vogt, F.M.; Barkhausen, J.; Thorns, C.; Bader, M.; Raasch, W. The brain renin-angiotensin system plays a crucial role in regulating body weight in diet-induced obesity in rats. Br. J. Pharmacol. 2016, 173, 1602–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campagnole-Santos, M.J.; Oliveira, M.F.; Braga, J.F.; Nadu, A.P.; Schreihofer, A.; Santos, R.A.; Campagnole-Santos, M.J. Increasing angiotensin-(1-7) levels in the brain attenuates metabolic syndrome-related risks in fructose-fed rats. Hypertension 2014, 63, 1078–1085. [Google Scholar] [CrossRef] [Green Version]
- Farag, E.; Sessler, D.I.; Ebrahim, Z.; Kurz, A.; Morgan, J.; Ahuja, S.; Maheshwari, K.; Doyle, D.J. The renin angiotensin system and the brain: New developments. J. Clin. Neurosci. 2017, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Cortes, C.; Touyz, R.M. Evolution of a New Class of Antihypertensive Drugs: Targeting the Brain Renin–angiotensin System. Hypertension 2020, 75, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, P.; Sigmund, C.D. How Is the Brain Renin–angiotensin System Regulated? Hypertension 2017, 70, 10–18. [Google Scholar] [CrossRef]
- Ren, L.; Lu, X.; Danser, A.H.J. Revisiting the Brain Renin–angiotensin System-Focus on Novel Therapies. Curr. Hypertens. Rep. 2019, 21, 28. [Google Scholar] [CrossRef] [Green Version]
- Wangensteen, R.; Segarra, A.B.; Ramirez-Sanchez, M.; De Gasparo, M.; Domínguez-Vías, G.; Banegas, I.; Vargas, F.; Vives, F.; Prieto, I. Influence of thyroid disorders on the kidney expression and plasma activity of aminopeptidase A. Endocr. Regul. 2015, 49, 68–72. [Google Scholar] [CrossRef]
- Ramírez, M.; Prieto, I.; Alba, F.; Vives, F.; Banegas, I.; De Gasparo, M. Role of central and peripheral aminopeptidase activities in the control of blood pressure: A working hypothesis. Heart Fail. Rev. 2008, 13, 339–353. [Google Scholar] [CrossRef]
- Marc, Y.; Llorens-Cortes, C. The role of the brain renin–angiotensin system in hypertension: Implications for new treatment. Prog. Neurobiol. 2011, 95, 89–103. [Google Scholar] [CrossRef]
- Gao, J.; Marc, Y.; Iturrioz, X.; Leroux, V.; Balavoine, F.; Llorens-Cortes, C. A new strategy for treating hypertension by blocking the activity of the brain renin–angiotensin system with aminopeptidase A inhibitors. Clin. Sci. 2014, 127, 135–148. [Google Scholar] [CrossRef]
- Marc, Y.; Hmazzou, R.; Balavoine, F.; Flahault, A.; Llorens-Cortes, C. Central antihypertensive effects of chronic treatment with RB150. J. Hypertens. 2018, 36, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Prieto, I.; Vargas, F.; Alba, F.; De Gasparo, M.; Ramirez, M. Angiotensinase activity in hypothalamus and pituitary of hypothyroid, euthyroid and hyperthyroid adult male rats. Horm. Metab. Res. 2003, 35, 279–281. [Google Scholar] [CrossRef]
- Villarejo, A.B.; Segarra, A.B.; Ramírez-Sánchez, M.; Banegas, I.; Wangensteen, R.; De Gasparo, M.; Cobo, J.; Alba, F.; Vives, F.; Prieto, I. Angiotensinase and vasopressinase activities in hypothalamus, plasma, and kidney after inhibition of angiotensin-converting enzyme: Basis for a new working hypothesis. Horm. Metab. Res. 2011, 44, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Prieto, I.; Martínez-Cañamero, M.; De Gasparo, M.; Luna, J.D.D.; Ramírez-Sánchez, M. Thyroid Disorders Change the Pattern of Response of Angiotensinase Activities in the Hypothalamus-Pituitary-Adrenal Axis of Male Rats. Front. Endocrinol. 2018, 9, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, R.L.; Hilzendeger, A.M.; Visniauskas, B.; Todiras, M.; Alenina, N.; Mori, M.A.; Araújo, R.C.; Nakaie, C.R.; Chagas, J.R.; Carmona, A.K.; et al. High aminopeptidase A activity contributes to blood pressure control in ob/ob mice by AT2 receptor-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H437–H445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrocsyova, V.; Balážová, L.; Kršková, K.; Horváthová, Ľ.; Olszanecki, R.; Suski, M.; Zórad, Š. Obesity and aging affects skeletal muscle renin–angiotensin system and myosin heavy chain proportions in pre-diabetic Zucker rats. J. Physiol. Biochem. 2019, 75, 351–365. [Google Scholar] [CrossRef]
- Arechaga, G.; Martínez, J.M.; Prieto, I.; Ramírez, M.J.; Sánchez, M.J.; Alba, F.; De Gasparo, M.; Ramírez, M. Serum aminopeptidase A activity of mice is related to dietary fat saturation. J. Nutr. 2001, 131, 1177–1179. [Google Scholar] [CrossRef] [Green Version]
- Segarra, A.B.; Ramírez-Sánchez, M.; Banegas, I.; Ruiz-Sanz, J.-I.; Ruiz-Larrea, M.; De Gasparo, M.; Martínez-Cañamero, M.; Vives, F.; Prieto, I. The profile of fatty acids in frontal cortex of rats depends on the type of fat used in the diet and correlates with neuropeptidase activities. Horm. Metab. Res. 2011, 43, 86–91. [Google Scholar] [CrossRef]
- Diéguez, C.; Vazquez, M.J.; Romero, A.; López, M.; Nogueiras, R. Hypothalamic control of lipid metabolism: Focus on leptin, ghrelin and melanocortins. Neuroendocrinology 2011, 94, 1–11. [Google Scholar] [CrossRef]
- Taher, J.; Farr, S.; Adeli, K. Central nervous system regulation of hepatic lipid and lipoprotein metabolism. Curr. Opin. Lipidol. 2017, 28, 32–38. [Google Scholar] [CrossRef]
- Cabassi, A.; Coghi, P.; Govoni, P.; Barouhiel, E.; Speroni, E.; Cavazzini, S.; Cantoni, A.M.; Scandroglio, R.; Fiaccadori, E. Sympathetic modulation by carvedilol and losartan reduces angiotensin II-mediated lipolysis in subcutaneous and visceral fat. J. Clin. Endocrinol. Metab. 2005, 90, 2888–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Le, G.-W.; Li, A.; Zheng, J.; Shi, Y. Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition 2006, 22, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Eu, C.H.A.; Lim, W.Y.A.; Ton, S.H.; Kadir, K.B.A. Glycyrrhizic acid improved lipoprotein lipase expression, insulin sensitivity, serum lipid and lipid deposition in high-fat diet-induced obese rats. Lipids Health Dis. 2010, 9, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maréchal, L.; Laviolette, M.; Rodrigue-Way, A.; Sow, B.; Brochu, M.; Caron, V.; Tremblay, A. The CD36-PPARγ Pathway in Metabolic Disorders. Int. J. Mol. Sci. 2018, 19, 1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granados-Principal, S.; Quiles, J.L.; Ramírez-Tortosa, C.; Ochoa, J.J.; Perez-Lopez, P.; Pulido-Moran, M.; Ramirez-Tortosa, M. Squalene ameliorates atherosclerotic lesions through the reduction of CD36 scavenger receptor expression in macrophages. Mol. Nutr. Food Res. 2012, 56, 733–740. [Google Scholar] [CrossRef]
- Bruce, C.R.; Hoy, A.J.; Turner, N.; Watt, M.J.; Allen, T.L.; Carpenter, K.; Cooney, G.J.; Febbraio, M.A.; Kraegen, E.W. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes 2009, 58, 550–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| CONTROL DIET | EVOO DIET | BUTTER DIET | ||
|---|---|---|---|---|
| Body Weight (BW.g) | 40.5 ± 0.66 | 39.8 ± 0.26 | 44.1 ±0.97 ab | p < 0.01 |
| Body Mass Index (BMI) | 0.38 ± 0.005 | 0.39 ± 0.009 | 0.41 ± 0.010 | ns |
| Heart weight (HW, g) | 0.22 ± 0.005 | 0.22± 0.006 | 0.24 ± 0.009 | ns |
| HW/BW (%) | 0.54 ± 0.007 | 0.53± 0.014 | 0.55 ± 0.01 | ns |
| Liver Weight (LW.g) | 1.54 ± 0.075 | 1.75 ± 0.072 | 1.67 ± 0.065 | ns |
| LW/BW (%) | 3.79 ± 0.145 | 4.32 ± 0.148 | 3.88 ± 0.089 ab | p < 0.05 |
| Visceral Adipose Tissue Weight (VATW.g) | 0.40 ± 0.069 | 0.56 ± 0.074 | 0.95 ± 0.199 ab | p < 0.05 |
| VATW/BW (%) | 1.08 ± 0.184 | 1.39 ± 0.183 | 2.13 ± 0.401 ab | p < 0.05 |
| Food Intake (g/day) | 3.4 ± 0.65 | 3.8 ± 0.41 a | 3.2 ± 0.26 ab | p < 0.05 |
| CONTROL DIET | EVOO DIET | BUTTER DIET | ||
|---|---|---|---|---|
| Fasting leptin (pg/mL) | 1686.0 ± 166.33 | 645.0 ± 80.11 a | 1646.0 ± 231.20 b | p < 0.01 |
| Postprandial leptin (pg/mL) | 1278.4 ± 149.85 | 3673.4 ± 476.03 | 5485.1 ± 1932.35 a | p < 0.05 |
| Fasting ghrelin (pg/mL) | 39.3 ± 9.29 | 173.4 ± 56.06 a | 31.6 ± 5.28 b | p < 0.05 |
| Postprandial ghrelin (pg/mL) | 10.4 ±2.68 | 8.8 ± 0.76 | 9.8 ± 2.89 | ns |
| CONTROL DIET | EVOO DIET | BUTTER DIET | |
|---|---|---|---|
| CD36 | 1 | 1.15 ± 0.116 | 0.72 ± 0.091 b** |
| FAS | 1 | 0.99 ± 0.111 | 1.03 ± 0.071 |
| CPT-1 | 1 | 0.97 ± 0.133 | 0.68 ± 0.083 a* |
| LPL | 1 | 1.01 ±0.111 | 0.73±0.107 |
| CONTROL DIET | EVOO DIET | BUTTER DIET | |
|---|---|---|---|
| CD36 | 1 | 0.41 ± 0.088 a** | 0.18 ± 0.038 a**b** |
| FAS | 1 | 1.33 ± 0.121 | 0.83±0.135 b** |
| CPT-1 | 1 | 1.00 ± 0.114 | 0.35± 0.061 a**b** |
| LPL | 1 | 0.71 ± 0.090 a** | 0.59 ± 0.112 a** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segarra, A.B.; Domínguez-Vías, G.; Redondo, J.; Martínez-Cañamero, M.; Ramírez-Sánchez, M.; Prieto, I. Hypothalamic Renin–Angiotensin System and Lipid Metabolism: Effects of Virgin Olive Oil versus Butter in the Diet. Nutrients 2021, 13, 480. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020480
Segarra AB, Domínguez-Vías G, Redondo J, Martínez-Cañamero M, Ramírez-Sánchez M, Prieto I. Hypothalamic Renin–Angiotensin System and Lipid Metabolism: Effects of Virgin Olive Oil versus Butter in the Diet. Nutrients. 2021; 13(2):480. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020480
Chicago/Turabian StyleSegarra, Ana Belén, Germán Domínguez-Vías, José Redondo, Magdalena Martínez-Cañamero, Manuel Ramírez-Sánchez, and Isabel Prieto. 2021. "Hypothalamic Renin–Angiotensin System and Lipid Metabolism: Effects of Virgin Olive Oil versus Butter in the Diet" Nutrients 13, no. 2: 480. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020480