Transepithelial Anti-Neuroblastoma Response to Kale among Four Vegetable Juices Using In Vitro Model Co-Culture System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Juice Preparations
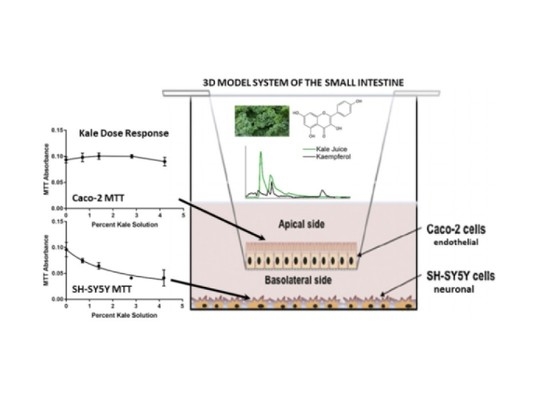
2.3. Co-Culture Plating and Treatments
2.4. TEER and Other Maturity Measures of the Caco-2Bbe1 Inserts
2.5. Comparative Role of Lactobacillus Fermentum
2.6. Automated Cell Counts by Cellometry
2.7. Metabolism of 3-(4,5-Dimethylthiazol-2yl)2,5-Diphenyl Tetrazolium Bromide
2.8. Reactive Oxygen Species Assay
2.9. Cell Cycle Analysis
2.10. Spectroscopic Measurement of the Juices
2.11. Chromatographic Measurement of the Juices
3. Results
3.1. Chemical Analysis of the Juices
3.1.1. Steady State Absorption and Fluorescence
3.1.2. Vibrational Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makkapati, S.; D’Agati, V.D.; Balsam, L. “Green Smoothie Cleanse” Causing Acute Oxalate Nephropathy. Am. J. Kidney Dis. 2018, 71, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zirpoli, G.R.; McCann, S.E.; Moysich, K.B.; Ambrosone, C.B.; Tang, L. Trends in Cruciferous Vegetable Consumption and Associations with Breast Cancer Risk: A Case-Control Study. Curr. Dev. Nutr. 2017, 1, e000448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qazi, A.; Pal, J.; Maitah, M.; Fulciniti, M.; Pelluru, D.; Nanjappa, P.; Lee, S.; Batchu, R.B.; Prasad, M.; Bryant, C.S.; et al. Anticancer activity of a broccoli derivative, sulforaphane, in barrett adenocarcinoma: Potential use in chemoprevention and as adjuvant in chemotherapy. Transl. Oncol. 2010, 3, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Casado, A. The Health Potential of Fruits and Vegetables Phytochemicals: Notable Examples. Crit. Rev. Food Sci. Nutr. 2016, 56, 1097–1107. [Google Scholar] [CrossRef]
- Manchali, S.; Murthy, K.N.C.; Patil, B.S. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Soundararajan, P.; Kim, J. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Antiproliferative Effect of Bioaccessible Fractions of Four Brassicaceae Microgreens on Human Colon Cancer Cells Linked to Their Phytochemical Composition. Antioxidants 2020, 9, 368. [Google Scholar] [CrossRef]
- Qizilbash, B.M. Effect of Curly Kale Brassica oleracea var. Sabellica on Viability of Cultured Mouse Melanoma Cell; Mississippi College: Clinton, MS, USA, 2015. [Google Scholar]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2019, 59, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Visioli, F. Polyphenols and health: Moving beyond antioxidants. J. Berry Res. 2012, 2, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Lomnitski, L.; Bergman, M.; Nyska, A.; Ben-Shaul, V.; Grossman, S. Composition, Efficacy, and Safety of Spinach Extracts. Nutr. Cancer 2003, 46, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Wu, Y.S.; Tunki, L.; Chellian, R.; Halmuthur, M.S.K.; Muller, S.; Pandy, V. What Has Come out from Phytomedicines and Herbal Edibles for the Treatment of Cancer? ChemMedChem 2018, 13, 1854–1872. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.L.; McMartin, K.E. Calcium Oxalate Monohydrate is Associated with Endothelial Cell Toxicity but Not with Reactive Oxygen Species Accumulation. Cardiovasc. Toxicol. 2020, 20, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Akinwumi, B.; Bordun, K.-A.; Anderson, H. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y. Comparison of nutritional compositions and antioxidant activities of building blocks in shinseoncho and kale green vegetable juices. Prev. Nutr. Food Sci. 2012, 17, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Speciale, A.; Chirafisi, J.; Saija, A.; Cimino, F. Nutritional antioxidants and adaptive cell responses: An update. Curr. Mol. Med. 2011, 11, 770–789. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Stephenson, K.K.; Wade, K.L.; Liu, H.; Fahey, J.W. Structure-activity analysis of flavonoids: Direct and indirect antioxidant, and antiinflammatory potencies and toxicities. Nutr. Cancer 2013, 65, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, R.; Tsao, R. Anthocyanin-rich phenolic extracts of purple root vegetables inhibit pro-inflammatory cytokines induced by H2O2 and enhance antioxidant enzyme activities in Caco-2 cells. J. Funct. Foods 2016, 22, 363–375. [Google Scholar] [CrossRef]
- Khuda-Bukhsh, A.R.; Das, S.; Saha, S.K. Molecular approaches toward targeted cancer prevention with some food plants and their products: Inflammatory and other signal pathways. Nutr. Cancer 2014, 66, 194–205. [Google Scholar] [CrossRef]
- Delle Monache, S.; Sanità, P.; Trapasso, E.; Ursino, M.R.; Dugo, P.; Russo, M.; Ferlazzo, N.; Calapai, G.; Angelucci, A.; Navarra, M. Mechanisms underlying the anti-tumoral effects of Citrus Bergamia juice. PLoS ONE 2013, 8, e61484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalak, M.; Szwajgier, D.; Paduch, R.; Kukula-Koch, W.; Waśko, A.; Polak-Berecka, M. Fermented curly kale as a new source of gentisic and salicylic acids with antitumor potential. J. Funct. Foods 2020, 67, 103866. [Google Scholar] [CrossRef]
- Vanhanen, L.; Savage, G. Comparison of oxalate contents and recovery from two green juices prepared using a masticating juicer or a high speed blender. NFS J. 2015, 1, 20–23. [Google Scholar] [CrossRef] [Green Version]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.-N.T. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/ p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 2017, 8, 17216–17228. [Google Scholar] [CrossRef] [Green Version]
- Aravindan, N.; Jain, D.; Somasundaram, D.B.; Herman, T.S.; Aravindan, S. Cancer stem cells in neuroblastoma therapy resistance. Cancer Drug Resist. (Alhambra Calif.) 2019, 2, 948–967. [Google Scholar] [CrossRef] [Green Version]
- NavaneethaKrishnan, S.; Rosales, J.L.; Lee, K.-Y. ROS-Mediated Cancer Cell Killing through Dietary Phytochemicals. Oxid. Med. Cell. Longev. 2019, 2019, 9051542. [Google Scholar] [CrossRef]
- Shinn, E.H.; Broderick, G.; Fellman, B.; Johnson, A.; Wieland, E.; Moulder, S.; Symmans, W.F. Simulating Time-Dependent Patterns of Nonadherence by Patients with Breast Cancer to Adjuvant Oral Endocrine Therapy. JCO Clin. Cancer Inform. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Traka, M.H.; Mithen, R.F. Plant science and human nutrition: Challenges in assessing health-promoting properties of phytochemicals. Plant Cell 2011, 23, 2483–2497. [Google Scholar] [CrossRef] [Green Version]
- Van Ginkel, P.R.; Sareen, D.; Subramanian, L.; Walker, Q.; Darjatmoko, S.R.; Lindstrom, M.J.; Kulkarni, A.; Albert, D.M.; Polans, A.S. Resveratrol inhibits tumor growth of human neuroblastoma and mediates apoptosis by directly targeting mitochondria. Clin. Cancer Res. 2007, 13, 5162–5169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, K.; Mock, C.; Liang, D. Pre-formulation studies of resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.J.; Lee, S.H.; Sung, N.J. Inhibitory effect of whole strawberries, garlic juice or kale juice on endogenous formation of N-nitrosodimethylamine in humans. Cancer Lett. 2002, 182, 1–10. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or sulforaphane: Is it the source or dose that matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [Green Version]
- Kassie, F.; Uhl, M.; Rabot, S.; Grasl-Kraupp, B.; Verkerk, R.; Kundi, M.; Chabicovsky, M.; Schulte-Hermann, R.; Knasmüller, S. Chemoprevention of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ)-induced colonic and hepatic preneoplastic lesions in the F344 rat by cruciferous vegetables administered simultaneously with the carcinogen. Carcinogenesis 2003, 24, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.K.; Mithen, R.; Johnson, I.T. Effects of Brassica vegetable juice on the induction of apoptosis and aberrant crypt foci in rat colonic mucosal crypts in vivo. Carcinogenesis 2003, 24, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.G.; Kim, J.-H.; Son, M.-J.; Lee, E.-J.; Park, W.-D.; Kim, J.-B.; Lee, S.-P.; Lee, I.-S. Influence of extraction method on quality and functionality of broccoli juice. Prev. Nutr. food Sci. 2013, 18, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Brandi, G.; Schiavano, G.F.; Zaffaroni, N.; De Marco, C.; Paiardini, M.; Cervasi, B.; Magnani, M. Mechanisms of Action and Antiproliferative Properties of Brassica oleracea Juice in Human Breast Cancer Cell Lines. J. Nutr. 2005, 135, 1503–1509. [Google Scholar] [CrossRef] [Green Version]
- Olsen, H.; Grimmer, S.; Aaby, K.; Saha, S.; Borge, G.I.A. Antiproliferative effects of fresh and thermal processed green and red cultivars of curly kale (Brassica oleracea L. convar. acephala var. sabellica). J. Agric. Food Chem. 2012, 60, 7375–7383. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.-S.; Bornhorst, G.M.; Oteiza, P.I.; Mitchell, A.E. Assessing the fate and bioavailability of glucosinolates in kale (Brassica oleracea) using simulated human digestion and Caco-2 cell uptake models. J. Agric. Food Chem. 2019, 67, 9492–9500. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Shimizu, S.; Miyamoto, S.; Izumi, T.; Obata, A.; Kikuchi, M.; Terao, J. Unique uptake and transport of isoflavone aglycones by human intestinal Caco-2 cells: Comparison of isoflavonoids and flavonoids. J. Nutr. 2002, 132, 1956–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Cao, W.; Xia, M.; Pan, S.; Xu, X. Study of structure and permeability relationship of flavonoids in Caco-2 cells. Nutrients 2017, 9, 1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visanji, J.M.; Duthie, S.J.; Pirie, L.; Thompson, D.G.; Padfield, P.J. Dietary isothiocyanates inhibit Caco-2 cell proliferation and induce G 2/M phase cell cycle arrest, DNA damage, and G2/M checkpoint activation. J. Nutr. 2004, 134, 3121–3126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louzao, M.C.; Fernández, D.A.; Abal, P.; Fraga, M.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Diarrhetic effect of okadaic acid could be related with its neuronal action: Changes in neuropeptide Y. Toxicol. Lett. 2015, 237, 151–160. [Google Scholar] [CrossRef]
- Van Groningen, T.; Koster, J.; Valentijn, L.J.; Zwijnenburg, D.A.; Akogul, N.; Hasselt, N.E.; Broekmans, M.; Haneveld, F.; Nowakowska, N.E.; Bras, J.; et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat. Genet. 2017, 49, 1261–1266. [Google Scholar] [CrossRef]
- Boeva, V.; Louis-Brennetot, C.; Peltier, A.; Durand, S.; Pierre-Eugène, C.; Raynal, V.; Etchevers, H.C.; Thomas, S.; Lermine, A.; Daudigeos-Dubus, E.; et al. Heterogeneity of neuroblastoma cell identity defined by transcriptional circuitries. Nat. Genet. 2017, 49, 1408–1413. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Lazcoz, P.; Celay, J.; Blanco, I.; Encio, I.; Fan, X.; Castresana, J. Differential capacity of neuroblastoma cell lines to produce neurospheres in vitro. Cancer Res. 2008, 68, 3781 LP. [Google Scholar]
- Abou-Antoun, T.J.; Nazarian, J.; Ghanem, A.; Vukmanovic, S.; Sandler, A.D. Molecular and functional analysis of anchorage independent, treatment-evasive neuroblastoma tumorspheres with enhanced malignant properties: A possible explanation for radio-therapy resistance. PLoS ONE 2018, 13, e0189711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Piletz, J.E. Kale, A Potential Treatment for Neuroblastoma Shown by Killing SH-SY5Y Cells. In Proceedings of the Mississippi Academy of Sciences Annual Meeting, Hattiesburg, MS, USA, 23–24 February 2017; Volume 62, p. 48. [Google Scholar]
- Piletz, J.E.; Drivon, J.; Eisenga, J.; Buck, W.; Yen, S.; McLin, M.; Meruvia, W.; Amaral, C.; Brue, K. Human Cells Grown With or Without Substitutes for Fetal Bovine Serum. Cell Med. 2018, 10, 215517901875514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Trinh, N.; Dang, N.D.-P.; Tran, D.H.; Van Pham, P. Taraxacum officinale dandelion extracts efficiently inhibited the breast cancer stem cell proliferation. Biomed. Res. Ther. 2016, 3, 733–741. [Google Scholar] [CrossRef]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Schiller, C.; Frohlich, C.-P.; Giessmann, T.; Siegmund, W.; Monnikes, H.; Hosten, N.; Weitshies, W. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2005, 22, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Jarosova, V.; Vesely, O.; Doskocil, I.; Tomisova, K.; Marsik, P.; Jaimes, J.D.; Smejkal, K.; Kloucek, P.; Havlik, J. Metabolism of cis- and trans-Resveratrol and Dihydroresveratrol in an Intestinal Epithelial Model. Nutrients 2020, 12, 595. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.K.; Seo, D.H.; Sung, J.M.; Ku, S.K.; Jeon, K.H.; Kim, Y.B.; Choi, Y.S. Study of optimization of natural nitrite source production from spinach. Korean J. Food Sci. Technol. 2017, 49, 459–461. [Google Scholar] [CrossRef]
- Maioli, E.; Torricelli, C.; Fortino, V.; Carlucci, F.; Tommassini, V.; Pacini, A. Critical appraisal of the MTT assay in the presence of rottlerin and uncouplers. Biol. Proced. Online 2009, 11, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Bernhard, D.; Schwaiger, W.; Crazzolara, R.; Tinhofer, I.; Kofler, R.; Csordas, A. Enhanced MTT-reducing activity under growth inhibition by resveratrol in CEM-C7H2 lymphocytic leukemia cells. Cancer Lett. 2003, 195, 193–199. [Google Scholar] [CrossRef]
- Bruggisser, R.; von Daeniken, K.; Jundt, G.; Schaffner, W.; Tullberg-Reinert, H. Interference of Plant Extracts, Phytoestrogens and Antioxidants with the MTT Tetrazolium Assay. Planta Med. 2002, 68, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, D.; Ari, F.; Ulukaya, E. The MTT viability assay yields strikingly false-positive viabilities although the cells are killed by some plant extracts. Turk. J. Biol. Turk Biyol. Derg. 2017, 41, 919–925. [Google Scholar] [CrossRef]
- Li, B.-X.; Zhang, M.-C.; Luo, C.-L.; Yang, P.; Li, H.; Xu, H.-M.; Xu, H.-F.; Shen, Y.-W.; Xue, A.-M.; Zhao, Z.-Q. Effects of RNA interference-mediated gene silencing of JMJD2A on human breast cancer cell line MDA-MB-231 in vitro. J. Exp. Clin. Cancer Res. 2011, 30, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Khayal, K.; Alafeefy, A.; Vaali-Mohammed, M.-A.; Mahmood, A.; Zubaidi, A.; Al-Obeed, O.; Khan, Z.; Abdulla, M.; Ahmad, R. Novel derivative of aminobenzenesulfonamide (3c) induces apoptosis in colorectal cancer cells through ROS generation and inhibits cell migration. BMC Cancer 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, S.; Zietz, M.; Schreiner, M.; Rohn, S.; Kroh, L.W.; Krumbein, A. Identification of complex, naturally occurring flavonoid glycosides in kale (Brassica oleracea var. sabellica) by high-performance liquid chromatography diode-array detection/electrospray ionization multi-stage mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.; Aaby, K.; Borge, G.I.A. Characterization and quantification of flavonoids and hydroxycinnamic acids in curly kale (Brassica oleracea L. convar. acephala var. sabellica) by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2009, 57, 2816–2825. [Google Scholar] [CrossRef]
- Putt, K.K.; Pei, R.; White, H.M.; Bolling, B.W. Yogurt inhibits intestinal barrier dysfunction in Caco-2 cells by increasing tight junctions. Food Funct. 2017, 8, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, B.; Sahihi, M.; Dehkhodaei, M.; Kelly, D.; Arany, I. Differential roles of 3-Hydroxyflavone and 7-Hydroxyflavone against nicotine-induced oxidative stress in rat renal proximal tubule cells. PLoS ONE 2017, 12, e0179777. [Google Scholar] [CrossRef] [Green Version]
- Kniseley, R.N.; Hirschmann, R.P.; Fassel, V.A. The infrared spectra of alkyl isothiocyanates. Spectrochim. Acta Part A Mol. Spectrosc. 1967, 23, 109–127. [Google Scholar] [CrossRef]
- Svátek, E.; Zahradník, R.; Kjær, A.; Sömme, R.; Stenhagen, E.; Palmstierna, H. Absorption Spectra of Alkyl isoThiocyanates and N-Alkyl Monothiocarbamates. Acta Chem. Scand. 1959, 13, 442–455. [Google Scholar] [CrossRef]
- Favaro, L.; Balcão, V.; Rocha, L.; Silva, E.; Oliveira, J., Jr.; Vila, M.; Tubino, M.; Favaro, L.I.L.; Balcão, V.M.; Rocha, L.K.H.; et al. Physicochemical Characterization of a Crude Anthocyanin Extract from the Fruits of Jussara (Euterpe edulis Martius): Potential for Food and Pharmaceutical Applications. J. Braz. Chem. Soc. 2018, 29, 2072–2088. [Google Scholar] [CrossRef]
- Caramês, E.T.S.; Alamar, P.D.; Poppi, R.J.; Pallone, J.A.L. Rapid Assessment of Total Phenolic and Anthocyanin Contents in Grape Juice Using Infrared Spectroscopy and Multivariate Calibration. Food Anal. Methods 2017, 10, 1609–1615. [Google Scholar] [CrossRef]
- Holen, B. Rapid separation of free sterols by reversed-phase high performance liquid chromatography. J. Am. Oil Chem. Soc. 1985, 62, 1344–1346. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT Food Sci. Technol. 2006, 39, 1155–1162. [Google Scholar] [CrossRef]
- Fujioka, N.; Fritz, V.; Upadhyaya, P.; Kassie, F.; Hecht, S.S. Research on cruciferous vegetables, indole-3-carbinol, and cancer prevention: A tribute to Lee W. Wattenberg. Mol. Nutr. Food Res. 2016, 60, 1228–1238. [Google Scholar] [CrossRef]
- Müller, H. Determination of the carotenoid content in selected vegetables and fruit by HPLC and photodiode array detection. Z. Leb. Und Forsch. A 2002, 204, 88–94. [Google Scholar] [CrossRef]
- Hahn, C.; Müller, A.; Kuhnert, N.; Albach, D. Diversity of Kale (Brassica oleracea var. sabellica): Glucosinolate Content and Phylogenetic Relationships. J. Agric. Food Chem. 2016. [Google Scholar] [CrossRef]
- Cartea, M.E.; Lema, M.; Francisco, M.; Velasco, P. Basic information on vegetable Brassica crops. In Genetics, Genomics and Breeding of Vegetable BRASSICAS; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–33. ISBN 978-1-57808-706-8. [Google Scholar]
- Farag, M.A.; Motaal, A.A.A. Sulforaphane composition, cytotoxic and antioxidant activity of crucifer vegetables. J. Adv. Res. 2010, 1, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Díaz, T.G.; Merás, I.D.; Rodríguez, D.A. Determination of resveratrol in wine by photochemically induced second-derivative fluorescence coupled with liquid-liquid extraction. Anal. Bioanal. Chem. 2007, 387, 1999–2007. [Google Scholar] [CrossRef]
- McCormack, D.; McFadden, D. A review of pterostilbene antioxidant activity and disease modification. Oxid. Med. Cell. Longev. 2013, 2013, 575482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigolio, R.; Miloso, M.; Nicolini, G.; Villa, D.; Scuteri, A.; Simone, M.; Tredici, G. Resveratrol interference with the cell cycle protects human neuroblastoma SH-SY5Y cell from paclitaxel-induced apoptosis. Neurochem. Int. 2005, 46, 205–211. [Google Scholar] [CrossRef]
- Tan, K.-T.; Chen, P.-W.; Li, S.; Ke, T.-M.; Lin, S.-H.; Yang, C.-C. Pterostilbene inhibits lung squamous cell carcinoma growth in vitro and in vivo by inducing S phase arrest and apoptosis. Oncol. Lett. 2019, 18, 1631–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ushida, Y.; Boonyapichest, C.; Suganuma, H.; Tanaka, M.; Matsui, T. Paracellular transport of sulforaphane across Caco-2 cell monolayers. Food Sci. Technol. Res. 2016, 22, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Buga, G.M.; Wei, L.H.; Bauer, P.M.; Fukuto, J.M.; Ignarro, L.J. NG-hydroxy-L-arginine and nitric oxide inhibit Caco-2 tumor cell proliferation by distinct mechanisms. Am. J. Physiol. 1998, 275, R1256–R1264. [Google Scholar] [CrossRef] [Green Version]
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef] [Green Version]
- Cencic, A.; Langerholc, T. Functional cell models of the gut and their applications in food microbiology—A review. Int. J. Food Microbiol. 2010, 141 (Suppl. S1), S4–S14. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chang, S.J.; Wang, M.Y.; Chen, Y.L.; Huang, T.Y. Growth Inhibition and Apoptosis of Neuroblastoma Cells Through ROS-Independent MEK/ERK Activation by Sulforaphane. Cell Biochem. Biophys. 2013, 66, 765–774. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, B.; Luo, F.; Liu, J.; Yang, T. Quercetin protects neuroblastoma SH-SY5Y cells against oxidative stress by inhibiting expression of Krüppel-like factor 4. Neurosci. Lett. 2012, 527, 115–120. [Google Scholar] [CrossRef]
- Sun, X.; Du, M.; Navarre, D.A.; Zhu, M.-J. Purple Potato Extract Promotes Intestinal Epithelial Differentiation and Barrier Function by Activating AMP-activated Protein Kinase. Mol. Nutr. Food Res. 2018, 62, 1700536. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Arnao, M.B. Hydrophilic and lipophilic antioxidant activity in different leaves of three lettuce varieties. Int. J. Food Prop. 2005, 8, 521–528. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Yáñez, J.A.; Roupe, K.A.; Davies, N.M. High-performance liquid chromatographic analysis of pterostilbene in biological fluids using fluorescence detection. J. Pharm. Biomed. Anal. 2007, 43, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Nikhil, K.; Sharan, S.; Chakraborty, A.; Roy, P. Pterostilbene-Isothiocyanate Conjugate Suppresses Growth of Prostate Cancer Cells Irrespective of Androgen Receptor Status. PLoS ONE 2014, 9, e93335. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; García-Carmona, F. Aggregation State and pKa Values of (E)-Resveratrol as Determined by Fluorescence Spectroscopy and UV−Visible Absorption. J. Agric. Food Chem. 2008, 56, 7600–7605. [Google Scholar] [CrossRef]
- Poutaraud, A.; Latouche, G.; Martins, S.; Meyer, S.; Merdinoglu, D.; Cerovic, Z. Fast and Local Assessment of Stilbene Content in Grapevine Leaf by in Vivo Fluorometry. J. Agric. Food Chem. 2007, 55, 4913–4920. [Google Scholar] [CrossRef]
- Sengupta, B.; Pahari, B.; Blackmon, L.; Sengupta, P.K. Prospect of bioflavonoid fisetin as a quadruplex DNA ligand: A biophysical approach. PLoS ONE 2013, 8, e65383. [Google Scholar] [CrossRef] [Green Version]
- Bhagwat, S.; Haytowitz, D.B. USDA Database for the Flavonoid Content of Selected Foods; US Department of Agriculture: Beltsville, MD, USA, 2016.
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional ingredients from Brassicaceae species: Overview and perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef] [Green Version]
- Willenberg, I.; Michael, M.; Wonik, J.; Bartel, L.C.; Empl, M.T.; Schebb, N.H. Investigation of the absorption of resveratrol oligomers in the Caco-2 cellular model of intestinal absorption. Food Chem. 2015, 167, 245–250. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, Z.; Zhou, W.; Liu, J.; Zhang, Q.; Xia, J.; Liu, J.; Chen, N.; Li, M.; Zhu, R. Resveratrol inhibits proliferation in human colorectal carcinoma cells by inducing G1/S-phase cell cycle arrest and apoptosis through caspase/cyclin-CDK pathways. Mol. Med. Rep. 2014, 10, 1697–1702. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, A.; Talwar, P.; D’Hellencourt, C.L.; Ravanan, P. IRE1α is critical for Kaempferol-induced neuroblastoma differentiation. FEBS J. 2019, 286, 1375–1392. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tseng, S.-H.; Lai, H.-S.; Chen, W.-J. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery 2004, 136, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Joseph, C.; Ghosh, S.; Agarwal, A.; Mishra, M.K.; Sen, E. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol. Cancer Ther. 2007, 6, 2544–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Heerden, J.; Kruger, M. Management of neuroblastoma in limited-resource settings. World J. Clin. Oncol. 2020, 11, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Sagir, A.; Schmitt, M.; Dilger, K.; Häussinger, D. Inhibition of cytochrome P450 3A: Relevant drug interactions in gastroenterology. Digestion 2003, 68, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K. Targeted isotretinoin in neuroblastoma: Kinetics, genetics, or absorption. Clin. Cancer Res. 2013, 19, 311–313. [Google Scholar] [CrossRef] [Green Version]











| Treatment and Dose | % of Cells in S Phase Arrest (n = 2 per Dose) |
|---|---|
| PBS low/high | 8.9/6.4 (AVG of 4 = 7.7) |
| Kale low/high | 10.5/11.0 (AVG of 4 = 10.7 *) |
| Dandelion low/high | 8.1/6.3 (AVG of 4 = 7.2) |
| Lettuce low/high | 8.9/5.9 (AVG of 4 = 7.4) |
| Spinach low/high | 8.5/- * (AVG of 2 = 8.5) |
| Possible Vibrations | Vibrational Frequencies of Vegetable Juice (cm−1) | |||
|---|---|---|---|---|
| Kale | Dandelion | Spinach | Lettuce | |
| Aromatic –C=C stretching –OH stretching symmetrical and asymmetrical stretching vibration for the carboxyl ion (COO–) indicating the existence of carboxylic acid, ester, or carbonyl groups | B | b | B | 1616 m |
| Part of isothiocyanate signature, deformations and stretching of aromatic C=C vibration of the aromatic rings. Also, in =C–O–C group of flavonoids | 1560 s | 1580 m | 1560 m | 1513 m |
| –O–H in plane bending in polyphenols deformation vibration of the carbon-carbon bonds in the phenolic group | 1393 vs | 1393 s | 1393 m | 1393 m |
| C–O stretching of acid groups bending vibrations of –CH3 or –CH2 groups in carboxylic acid, with more of isothiocyanate signature (–N=C=S). Also, an oxalate signature (1310 cm−1). | 1330 b,vw | 1330 b,vw | 1322 s, 1310 s | 1322 s |
| stretching vibration of the aromatic rings and =C–O–C group of flavonoids C–N stretching | 1053 s | 1060 vs | 1056 m | 1056 s |
| Sulforaphane signature (assigned to the S=O bond), the prototypic isothiocyanate | 1033 vs | 1033 m | 1033 s | 1033 vs |
| Reported Status | Sulforaphane | Kaempferol | Resveratrol/Pterostilbene |
|---|---|---|---|
| Concentration greater in kale than other three vegetables | Yes [80] | Yes [101] | Levels not reported [101] |
| Concentration in green curly kale | 608 mg/100 g [36] * | 46.8 mg/100 g [101] | microgram range [102] |
| Bio-accessible during digestion | 34.1% [36] * Moderate | 98.6% [46] High | “Low” [18] |
| Transported across Caco-2 inserts (Papp, × 10−6 cm/s) | 31.2 [88] (good) | 5.9 [46] (acceptable) | 11.9 [103] (moderate) |
| Effect on Caco-2 monocultures | Inhibits [47] | None [46] | Inhibits [104] |
| Effect on neuroblastoma monocultures Growth | G2/M arrest [47] **, mainly cytotoxic [92] | S arrest, mainly cytostatic [105] | S arrest, mainly cytotoxic [106] |
| ROS Effect in neuroblastoma | No change [81] | Increased [107] *** | Concentration dependent ↑↓ [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piletz, J.E.; Mao, Y.; Roy, D.; Qizilbash, B.; Nkamssi, E.; Weir, E.; Graham, J.; Emmanuel, M.; Iqbal, S.; Brue, K.; et al. Transepithelial Anti-Neuroblastoma Response to Kale among Four Vegetable Juices Using In Vitro Model Co-Culture System. Nutrients 2021, 13, 488. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020488
Piletz JE, Mao Y, Roy D, Qizilbash B, Nkamssi E, Weir E, Graham J, Emmanuel M, Iqbal S, Brue K, et al. Transepithelial Anti-Neuroblastoma Response to Kale among Four Vegetable Juices Using In Vitro Model Co-Culture System. Nutrients. 2021; 13(2):488. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020488
Chicago/Turabian StylePiletz, John E., Yuhan Mao, Debarshi Roy, Bilal Qizilbash, Eurielle Nkamssi, Enleyona Weir, Jessica Graham, Mary Emmanuel, Suwaira Iqbal, Kellie Brue, and et al. 2021. "Transepithelial Anti-Neuroblastoma Response to Kale among Four Vegetable Juices Using In Vitro Model Co-Culture System" Nutrients 13, no. 2: 488. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020488