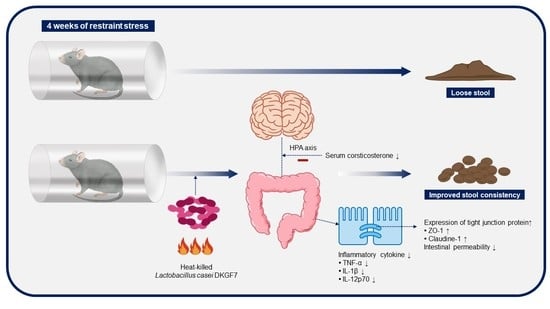
Effect of Heat-Killed Lactobacillus casei DKGF7 on a Rat Model of Irritable Bowel Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Preparation of the Heat-Killed Bacterial Strain
2.3. Serum Corticosterone Levels
2.4. Measurement of Inflammatory Cytokines in Colon Tissues
2.5. Expression of Tight Junction Proteins Using Immunohistochemistry
2.6. Statistical Analysis
3. Results
3.1. Body Weight
3.2. Stool Consistency
3.3. Serum Level of Corticosterone
3.4. Expression of Inflammatory Cytokines in Colon Tissues
3.5. Expression of Tight Junction Proteins
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.H.; Jung, H.K.; Kim, H.J.; Koo, H.S.; Kwon, Y.H.; Shin, H.D.; Lim, H.C.; Shin, J.E.; Kim, S.E.; Cho, D.H.; et al. Clinical practice guidelines for irritable bowel syndrome in korea, 2017. J. Neurogastroenterol. Motil. 2018, 24, 197–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef] [Green Version]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The microbiome and irritable bowel syndrome—A review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Koloski, N.A.; Jones, M.; Kalantar, J.; Weltman, M.; Zaguirre, J.; Talley, N.J. The brain-gut pathway in functional gastrointestinal disorders is bidirectional: A 12-year prospective population-based study. Gut 2012, 61, 1284–1290. [Google Scholar] [CrossRef]
- Ford, A.C.; Talley, N.J. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: A systematic review. J. Gastroenterol. 2011, 46, 421–431. [Google Scholar] [CrossRef]
- Gwee, K.A.; Collins, S.M.; Read, N.W.; Rajnakova, A.; Deng, Y.; Graham, J.C.; McKendrick, M.W.; Moochhala, S.M. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut 2003, 52, 523–526. [Google Scholar] [CrossRef]
- Marshall, J.K.; Thabane, M.; Garg, A.X.; Clark, W.; Meddings, J.; Collins, S.M.; WEL Investigators. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in walkerton, Ontario. Aliment. Pharmacol. Ther. 2004, 20, 1317–1322. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Gobbetti, M.; Cagno, R.D.; De Angelis, M. Functional microorganisms for functional food quality. Crit. Rev. Food Sci. Nutr. 2010, 50, 716–727. [Google Scholar] [CrossRef]
- Kataria, J.; Li, N.; Wynn, J.L.; Neu, J. Probiotic microbes: Do they need to be alive to be beneficial? Nutr. Rev. 2009, 67, 546–550. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Zang, Z.L.; Choi, E.Y.; Shin, H.K.; Ji, G.E. Cytoskeleton reorganization and cytokine production of macrophages by bifidobacterial cells and cell-free extracts. J. Microbiol. Biotechnol. 2002, 12, 398–405. [Google Scholar]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [Green Version]
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Dale, H.F.; Rasmussen, S.H.; Asiller, Ö.; Lied, G.A. Probiotics in irritable bowel syndrome: An up-to-date systematic review. Nutrients 2019, 11, 2048. [Google Scholar] [CrossRef] [Green Version]
- Wagner, R.D.; Pierson, C.; Warner, T.; Dohnalek, M.; Hilty, M.; Balish, E. Probiotic effects of feeding heat-killed lactobacillus acidophilus and lactobacillus casei to candida albicans-colonized immunodeficient mice. J. Food Prot. 2000, 63, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Russell, W.M.; Douglas-escobar, M.; Hauser, N.; Lopez, M.; Neu, J. Live and heat-killed Lactobacillus rhamnosus GG: Effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr. Res. 2009, 66, 203–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorjão, A.L.; de Oliveira, F.E.; Leão, M.V.; Carvalho, C.A.; Jorge, A.O.; de Oliveira, L.D. Live and heat-killed lactobacillus rhamnosus atcc 7469 may induce modulatory cytokines profiles on macrophages raw 264.7. ScientificWorldJournal 2015, 2015, 716749. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Kim, H.S.; Yoo, J.S.; Cho, Y.A.; Kim, C.H. Antioxidant activity of lactic acid bacteria isolated from Korean traditional food kimchi. J. Dairy Sci. Biotechnol. 2020, 38, 89–97. [Google Scholar] [CrossRef]
- Baick, S.C.; Kim, C.H. Assessment of characteristics and functional properties of lactobacillus species isolated from kimchi for dairy use. Korean J. Food Sci. Anim. Resour. 2015, 35, 339–349. [Google Scholar] [CrossRef]
- Oh, J.H.; Jang, Y.S.; Kang, D.; Chang, D.K.; Min, Y.W. Efficacy and safety of new lactobacilli probiotics for unconstipated irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. Nutrients 2019, 11, 2887. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.G.; Min, Y.W.; Lee, C.; Hong, S.N.; Won, J.Y.; Jang, J.A.; Kim, C.H.; Chang, D.K. Effects of novel probiotics in a murine model of irritable bowel syndrome. Korean J. Gastroenterol. 2020, 75, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, G.; Lee, S.; Min, Y.W.; Jang, Y.S.; Park, S.Y.; Kim, C.H.; Lee, C.; Hong, S.N.; Chang, D.K. Effect of a synbiotic containing Lactobacillus paracasei and Opuntia humifusa on a murine model of irritable bowel syndrome. Nutrients 2020, 12, 3205. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.T. Safety of probiotics: Translocation and infection. Nutr. Rev. 2008, 66, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, A.; Takahashi, S.; Ito, Y.; Ohishi, Y.; Tsukamoto, K.; Nanba, Y.; Ito, N.; Kakiuchi, S.; Saitoh, A.; Morotomi, M.; et al. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J. Pediatr. 2010, 156, 679–681. [Google Scholar] [CrossRef]
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L. Probiotic use in clinical practice: What are the risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264, quiz 1446–1447. [Google Scholar] [CrossRef] [PubMed]
- Petrof, E.O.; Dhaliwal, R.; Manzanares, W.; Johnstone, J.; Cook, D.; Heyland, D.K. Probiotics in the critically ill: A systematic review of the randomized trial evidence. Crit. Care Med. 2012, 40, 3290–3302. [Google Scholar] [CrossRef]
- van Reenen, C.A.; Dicks, L.M. Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: What are the possibilities? A review. Arch. Microbiol. 2011, 193, 157–168. [Google Scholar] [CrossRef]
- Deshpande, G.; Athalye-Jape, G.; Patole, S. Para-probiotics for preterm neonates-the next frontier. Nutrients 2018, 10, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (Tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, N.; Caicedo, R.; Neu, J. Alive and dead lactobacillus rhamnosus gg decrease tumor necrosis factor-alpha-induced interleukin-8 production in caco-2 cells. J. Nutr. 2005, 135, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Ramírez, L.M.; Pérez-Solano, R.A.; Castañón-Alonso, S.L.; Moreno Guerrero, S.S.; Ramírez Pacheco, A.; García Garibay, M.; Eslava, C. Probiotic lactobacillus strains stimulate the inflammatory response and activate human macrophages. J. Immunol. Res. 2017, 2017, 4607491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with lps. Evid. Based Complement. Alternat. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of surface exopolysaccharides from Bifidobacterium and lactobacillus within the intestinal environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef] [Green Version]
- Lopetuso, L.; Graziani, C.; Guarino, A.; Lamborghini, A.; Masi, S.; Stanghellini, V. Gelatin tannate and Tyndallized probiotics: A novel approach for treatment of diarrhea. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 873–883. [Google Scholar] [PubMed]
- Miyauchi, E.; Morita, H.; Tanabe, S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J. Dairy Sci. 2009, 92, 2400–2408. [Google Scholar] [CrossRef]
- Dinan, T.G.; Quigley, E.M.; Ahmed, S.M.; Scully, P.; O’Brien, S.; O’Mahony, L.; O’Mahony, S.; Shanahan, F.; Keeling, P.W. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: Plasma cytokines as a potential biomarker? Gastroenterology 2006, 130, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wu, S.P.; Hu, Y.; Smith, D.E.; Wiley, J.W.; Hong, S. Corticosterone mediates stress-related increased intestinal permeability in a region-specific manner. Neurogastroenterol. Motil. 2013, 25, e127–e139. [Google Scholar] [CrossRef]
- Zhen, Y.; Chu, C.; Zhou, S.; Qi, M.; Shu, R. Imbalance of tumor necrosis factor-α, interleukin-8 and interleukin-10 production evokes barrier dysfunction, severe abdominal symptoms and psychological disorders in patients with irritable bowel syndrome-associated diarrhea. Mol. Med. Rep. 2015, 12, 5239–5245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitselou, A.; Grammeniatis, V.; Varouktsi, A.; Papadatos, S.S.; Katsanos, K.; Galani, V. Proinflammatory cytokines in irritable bowel syndrome: A comparison with inflammatory bowel disease. Intest. Res. 2020, 18, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Bertiaux-Vandaële, N.; Youmba, S.B.; Belmonte, L.; Lecleire, S.; Antonietti, M.; Gourcerol, G.; Leroi, A.M.; Déchelotte, P.; Ménard, J.F.; Ducrotté, P.; et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am. J. Gastroenterol. 2011, 106, 2165–2173. [Google Scholar] [CrossRef]
- Aragon, G.; Graham, D.B.; Borum, M.; Doman, D.B. Probiotic therapy for irritable bowel syndrome. Gastroenterol. Hepatol. N. Y. 2010, 6, 39–44. [Google Scholar] [PubMed]
- Ananta, E.; Knorr, D. Comparison of inactivation pathways of thermal or high pressure inactivated lactobacillus rhamnosus atcc 53103 by flow cytometry analysis. Food Microbiol. 2009, 26, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Tomita, S.; Mercenier, A.; Kleerebezem, M. Cell surface-associated compounds of probiotic lactobacilli sustain the strain-specificity dogma. Curr. Opin. Microbiol. 2013, 16, 262–269. [Google Scholar] [CrossRef]
- Wong, C.; Ustunol, Z. Mode of inactivation of probiotic bacteria affects interleukin 6 and interleukin 8 production in human intestinal epithelial-like caco-2 cells. J. Food Prot. 2006, 69, 2285–2288. [Google Scholar] [CrossRef]
- Ou, C.C.; Lin, S.L.; Tsai, J.J.; Lin, M.Y. Heat-killed lactic acid bacteria enhance immunomodulatory potential by skewing the immune response toward th1 polarization. J. Food Sci. 2011, 76, M260–M267. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Evangelista, S. Experimental models of irritable bowel syndrome and the role of the enteric neurotransmission. J. Clin. Med. 2018, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.B. Repeated restraint stress lowers the threshold for response to third ventricle crf administration. Horm. Behav. 2017, 89, 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-González, M.; Díaz-Zepeda, C.; Eyzaguirre-Velásquez, J.; González-Arancibia, C.; Bravo, J.A.; Julio-Pieper, M. Investigating gut permeability in animal models of disease. Front. Physiol. 2018, 9, 1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.L.; Villar, R.G.; Peterson, J.M.; Burks, T.F. Stress-induced changes in intestinal transit in the rat: A model for irritable bowel syndrome. Gastroenterology 1988, 94, 611–621. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, G.; Lee, S.; Min, Y.W.; Jang, Y.S.; Kim, H.S.; Kim, E.-J.; Park, S.-Y.; Kim, C.-H.; Chang, D.K. Effect of Heat-Killed Lactobacillus casei DKGF7 on a Rat Model of Irritable Bowel Syndrome. Nutrients 2021, 13, 568. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020568
Seong G, Lee S, Min YW, Jang YS, Kim HS, Kim E-J, Park S-Y, Kim C-H, Chang DK. Effect of Heat-Killed Lactobacillus casei DKGF7 on a Rat Model of Irritable Bowel Syndrome. Nutrients. 2021; 13(2):568. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020568
Chicago/Turabian StyleSeong, Gyeol, Seungbaek Lee, Yang Won Min, Yeon Sil Jang, Hong Seog Kim, Eui-Joong Kim, So-Young Park, Cheol-Hyun Kim, and Dong Kyung Chang. 2021. "Effect of Heat-Killed Lactobacillus casei DKGF7 on a Rat Model of Irritable Bowel Syndrome" Nutrients 13, no. 2: 568. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13020568