Vegan Diet Health Benefits in Metabolic Syndrome
Abstract
:1. Introduction
2. Search Results
3. Plant-Based Dietary Patterns and Environmental Impacts
Strength and Weakness of Vegan Diets
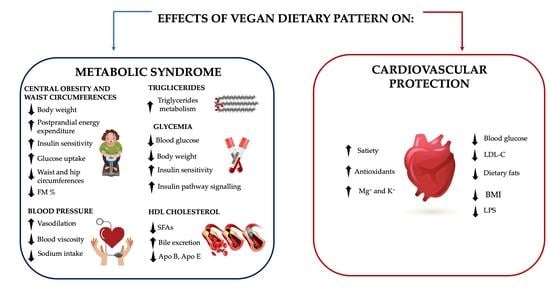
4. Metabolic Syndrome
4.1. Central Obesity and Waist Circumference
4.2. Blood Pressure
4.3. Lipid Metabolism
4.4. Glycemia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Society |
| AH | Arterial Hypertension |
| AHA/NHLBI | American Heart Association/National Heart, Lung and Blood Institute |
| AI | Adequate Intake |
| ALA | α-Linolenic Acid |
| ATP III-NCEP | Adult Treatment Panel III—National Cholesterol Education Program |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| CVD | Cardio-Vascular Disease |
| DASH | Dietary Approaches to Stop Hypertension |
| DBP | Diastolic Blood Pressure |
| DHA | Docosahexaenoic Acid |
| EASD | European Society for The Study of Diabetes |
| EPA | Eicosapentaenoic Acid |
| EVOO | Extra-Virgin Olive Oil |
| FAO | Food and Agriculture Organization of The United Nations |
| FV | Fish-Vegetarian |
| GHG | Green House Gas |
| HDL-C | High-Density Lipoprotein-Cholesterol |
| IDF | International Diabetes Federation |
| IF | Insoluble Fiber |
| IGF-1 | Insulin-like Growth Factor-1 |
| LDL-C | Low-Density Lipoprotein- Cholesterol |
| LOV | Lacto-Ovo-Vegetarian |
| LPS | Lipopolysaccharide |
| MD | Mediterranean Diet |
| MetS | Metabolic Syndrome |
| MUFA | Mono-Unsaturated Fatty Acid |
| NO | Nitric Oxide |
| PBD | Plant-Based Diet |
| PS | Phytosterol |
| PUFA | Poly-Unsaturated Fatty Acid |
| ROS | Reactive Oxygen Species |
| SBP | Systolic Blood Pressure |
| SF | Water-Soluble Fiber |
| SFA | Saturated Fatty Acid |
| T2DM | Type 2 Diabetes Mellitus |
| TC | Total Cholesterol |
| TG | Triglyceride |
| TLR4 | Toll-like Receptor 4 |
| TNF- α | Tumor Necrosis Factor-α |
References
- D’Almeida, K.S.M.; Ronchi Spillere, S.; Zuchinali, P.; Correa Souza, G. Mediterranean Diet and Other Dietary Patterns in Primary Prevention of Heart Failure and Changes in Cardiac Function Markers: A Systematic Review. Nutrients 2018, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Vogt, T.M.; Appel, L.J.; Obarzanek, E.; Moore, T.J.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Cutler, J.A.; Windhauser, M.M.; et al. Dietary Approaches to Stop Hypertension: Rationale, design, and methods. DASH Collaborative Research Group. J. Am. Diet. Assoc. 1999, 99, S12–S18. [Google Scholar] [CrossRef]
- Anand, S.S.; Hawkes, C.; de Souza, R.J.; Mente, A.; Dehghan, M.; Nugent, R.; Zulyniak, M.A.; Weis, T.; Bernstein, A.M.; Krauss, R.M.; et al. Food Consumption and its Impact on Cardiovascular Disease: Importance of Solutions Focused on the Globalized Food System: A Report From the Workshop Convened by the World Heart Federation. J. Am. Coll. Cardiol. 2015, 66, 1590–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreoli, A.; Lauro, S.; Di Daniele, N.; Sorge, R.; Celi, M.; Volpe, S.L. Effect of a moderately hypoenergetic Mediterranean diet and exercise program on body cell mass and cardiovascular risk factors in obese women. Eur. J. Clin. Nutr. 2008, 62, 892–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedehayir, O.; Riverola, C.; Velasquez, S.; Smidt, M. Diffusion of Vegan Food Innovations: A Dual-Market Perspective. In Responsible Consumption and Production; Leal Filho, W., Azul, A.M., Brandli, L., Özuyar, P.G., Wall, T., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–9. [Google Scholar] [CrossRef]
- Rocha, J.P.; Laster, J.; Parag, B.; Shah, N.U. Multiple Health Benefits and Minimal Risks Associated with Vegetarian Diets. Curr. Nutr. Rep. 2019, 8, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef] [PubMed]
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. A Plant-Based Dietary Intervention Improves Beta-Cell Function and Insulin Resistance in Overweight Adults: A 16-Week Randomized Clinical Trial. Nutrients 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchta, A.; Lebiedzinska, A.; Fijalkowski, M.; Galaska, R.; Kreft, E.; Toton, M.; Czaja, K.; Kozlowska, A.; Cwiklinska, A.; Kortas-Stempak, B.; et al. Impact of plant-based diet on lipid risk factors for atherosclerosis. Cardiol. J. 2016, 23, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Thakur, M.; Sangha, P.S.; Satti, A.; Shah, P.N. Cardiovascular Risk Reduction with Icosapent Ethyl: A Systematic Literature Review. Cureus 2020, 12, e10942. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-Based Diets Are Associated with a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef]
- Hever, J.; Cronise, R.J. Plant-based nutrition for healthcare professionals: Implementing diet as a primary modality in the prevention and treatment of chronic disease. J. Geriatr. Cardiol. 2017, 14, 355–368. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Hopwood, C.J.; Bleidorn, W.; Schwaba, T.; Chen, S. Health, environmental, and animal rights motives for vegetarian eating. PLoS ONE 2020, 15, e0230609. [Google Scholar] [CrossRef] [Green Version]
- Tuso, P.J.; Ismail, M.H.; Ha, B.P.; Bartolotto, C. Nutritional update for physicians: Plant-based diets. Perm. J. 2013, 17, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Levin, S.; Barnard, N.D. Vegetarian Dietary Patterns and Cardiovascular Disease. Prog. Cardiovasc. Dis. 2018, 61, 54–61. [Google Scholar] [CrossRef]
- Orlich, M.J.; Jaceldo-Siegl, K.; Sabate, J.; Fan, J.; Singh, P.N.; Fraser, G.E. Patterns of food consumption among vegetarians and non-vegetarians. Br. J. Nutr. 2014, 112, 1644–1653. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, K.; Brodowski, J.; Pokorska-Niewiada, K.; Szczuko, M. The Change in the Content of Nutrients in Diets Eliminating Products of Animal Origin in Comparison to a Regular Diet from the Area of Middle-Eastern Europe. Nutrients 2020, 12, 2986. [Google Scholar] [CrossRef]
- Wozniak, H.; Larpin, C.; de Mestral, C.; Guessous, I.; Reny, J.L.; Stringhini, S. Vegetarian, pescatarian and flexitarian diets: Sociodemographic determinants and association with cardiovascular risk factors in a Swiss urban population. Br. J. Nutr. 2020, 124, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Stewart, K.; Oda, K.; Batech, M.; Herring, R.P.; Fraser, G.E. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 292–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, B.J.; Anousheh, R.; Fan, J.; Jaceldo-Siegl, K.; Fraser, G.E. Vegetarian diets and blood pressure among white subjects: Results from the Adventist Health Study-2 (AHS-2). Public Health Nutr. 2012, 15, 1909–1916. [Google Scholar] [CrossRef] [Green Version]
- Petermann-Rocha, F.; Parra-Soto, S.; Gray, S.; Anderson, J.; Welsh, P.; Gill, J.; Sattar, N.; Ho, F.K.; Celis-Morales, C.; Pell, J.P. Vegetarians, fish, poultry, and meat-eaters: Who has higher risk of cardiovascular disease incidence and mortality? A prospective study from UK Biobank. Eur. Heart J. 2020. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Torres-Acosta, N.; O’Keefe, E.L.; Saeed, I.M.; Lavie, C.J.; Smith, S.E.; Ros, E. A Pesco-Mediterranean Diet With Intermittent Fasting: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Sacanella, E.; Ros, E. Should we all go pesco-vegetarian? Eur. Heart J. 2021. [Google Scholar] [CrossRef]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Huang, C.; Huang, J.; Tian, Y.; Yang, X.; Gu, D. Sugar sweetened beverages consumption and risk of coronary heart disease: A meta-analysis of prospective studies. Atherosclerosis 2014, 234, 11–16. [Google Scholar] [CrossRef]
- Hemler, E.C.; Hu, F.B. Plant-Based Diets for Cardiovascular Disease Prevention: All Plant Foods Are Not Created Equal. Curr. Atheroscler. Rep. 2019, 21, 18. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-Leon, A.M.; Sierra-Perez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Campo, M.; Urciuoli, S.; Marrone, G.; Noce, A.; Bernini, R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols from Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Bernini, R.; Noce, A.; Urciuoli, S.; Di Lauro, M.; Pietroboni Zaitseva, A.; Marrone, G.; Di Daniele, N. Potential Beneficial Effects of Extra Virgin Olive Oils Characterized by High Content in Minor Polar Compounds in Nephropathic Patients: A Pilot Study. Molecules 2020, 25, 4757. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington, DC: US Departments of Agriculture and Health and Human Services, 2015. Adv. Nutr. 2016, 7, 202–204. [Google Scholar] [CrossRef] [Green Version]
- Dietary Guidelines Advisory Committee. Washington (DC): US Department of Agriculture and US Department of Health and Human Services. Available online: https://health.gov/sites/default/files/2019-09/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf (accessed on 28 December 2020).
- Park, Y.M.; Steck, S.E.; Fung, T.T.; Zhang, J.; Hazlett, L.J.; Han, K.; Lee, S.H.; Kwon, H.S.; Merchant, A.T. Mediterranean diet, Dietary Approaches to Stop Hypertension (DASH) style diet, and metabolic health in U.S. adults. Clin. Nutr. 2017, 36, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.I.; Maiolo, C.; Iacopino, L.; Pepe, M.; Di Daniele, N.; De Lorenzo, A. The impact of body-weight components on forced spirometry in healthy italians. Lung 2002, 180, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Bocedi, A.; Campo, M.; Marrone, G.; Di Lauro, M.; Cattani, G.; Di Daniele, N.; Romani, A. A Pilot Study of a Natural Food Supplement as New Possible Therapeutic Approach in Chronic Kidney Disease Patients. Pharmaceuticals 2020, 13, 148. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Di Lauro, M.; Urciuoli, S.; Pietroboni Zaitseva, A.; Wilson Jones, G.; Di Daniele, N.; Romani, A. Cardiovascular Protection of Nephropathic Male Patients by Oral Food Supplements. Cardiovasc. Ther. 2020, 2020, 1807941. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Fabrini, R.; Dessi, M.; Bocedi, A.; Santini, S.; Rovella, V.; Pastore, A.; Tesauro, M.; Bernardini, S.; Di Daniele, N.; et al. Erythrocyte glutathione transferase activity: A possible early biomarker for blood toxicity in uremic diabetic patients. Acta Diabetol. 2014, 51, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N.; Marrone, G.; Di Lauro, M.; Di Daniele, F.; Palazzetti, D.; Guerriero, C.; Noce, A. Effects of caloric restriction diet on arterial hypertension and endothelial dysfunction. Nutrients 2021, 13, 274. [Google Scholar] [CrossRef]
- Tesauro, M.; Nistico, S.; Noce, A.; Tarantino, A.; Marrone, G.; Costa, A.; Rovella, V.; Di Cola, G.; Campia, U.; Lauro, D.; et al. The possible role of glutathione-S-transferase activity in diabetic nephropathy. Int. J. Immunopathol. Pharmacol. 2015, 28, 129–133. [Google Scholar] [CrossRef]
- Di Daniele, N. Association of Dietary Patterns with Metabolic Syndrome. Nutrients 2020, 12, 2840. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme. UNEP Year Book: Emerging Issues in Our Global Environment. 2012. Available online: http://www.unep.org/yearbook/2012 (accessed on 28 December 2020).
- Gill, M.; Feliciano, D.; Macdiarmid, J.; Smith, P. The environmental impact of nutrition transition in three case study countries. Food Secur. 2015. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, D.; Pimentel, M. Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 2003, 78, 660S–663S. [Google Scholar] [CrossRef]
- Baroni, L.; Cenci, L.; Tettamanti, M.; Berati, M. Evaluating the environmental impact of various dietary patterns combined with different food production systems. Eur. J. Clin. Nutr. 2007, 61, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarborough, P.; Appleby, P.N.; Mizdrak, A.; Briggs, A.D.; Travis, R.C.; Bradbury, K.E.; Key, T.J. Dietary greenhouse gas emissions of meat-eaters, fish-eaters, vegetarians and vegans in the UK. Clim. Chang. 2014, 125, 179–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.E.; Hamm, M.W.; Hu, F.B.; Abrams, S.A.; Griffin, T.S. Alignment of Healthy Dietary Patterns and Environmental Sustainability: A Systematic Review. Adv. Nutr. 2016, 7, 1005–1025. [Google Scholar] [CrossRef] [Green Version]
- Losasso, C.; Di Cesare, A.; Mastrorilli, E.; Patuzzi, I.; Cibin, V.; Eckert, E.M.; Fontaneto, D.; Vanzo, A.; Ricci, A.; Corno, G. Assessing antimicrobial resistance gene load in vegan, vegetarian and omnivore human gut microbiota. Int. J. Antimicrob. Agents 2018, 52, 702–705. [Google Scholar] [CrossRef]
- Dudek-Wicher, R.K.; Junka, A.; Bartoszewicz, M. The influence of antibiotics and dietary components on gut microbiota. Prz. Gastroenterol. 2018, 13, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Glick-Bauer, M.; Yeh, M.C. The health advantage of a vegan diet: Exploring the gut microbiota connection. Nutrients 2014, 6, 4822–4838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Di Daniele, F.; Ottaviani, E.; Wilson Jones, G.; Bernini, R.; Romani, A.; Rovella, V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients 2019, 11, 1073. [Google Scholar] [CrossRef] [Green Version]
- Paslakis, G.; Richardson, C.; Nohre, M.; Brahler, E.; Holzapfel, C.; Hilbert, A.; de Zwaan, M. Prevalence and psychopathology of vegetarians and vegans—Results from a representative survey in Germany. Sci. Rep. 2020, 10, 6840. [Google Scholar] [CrossRef] [Green Version]
- Rapporto Vegan Italia Osservatorio VEGANOK. 2017. Available online: https://www.osservatorioveganok.com/quanti-sono-i-vegani-in-italia/ (accessed on 26 January 2021).
- Takahashi, Y.; Sasaki, S.; Okubo, S.; Hayashi, M.; Tsugane, S. Blood pressure change in a free-living population-based dietary modification study in Japan. J. Hypertens. 2006, 24, 451–458. [Google Scholar] [CrossRef]
- Berkow, S.E.; Barnard, N. Vegetarian diets and weight status. Nutr. Rev. 2006, 64, 175–188. [Google Scholar] [CrossRef]
- Craig, W.J. Health effects of vegan diets. Am. J. Clin. Nutr. 2009, 89, 1627S–1633S. [Google Scholar] [CrossRef] [Green Version]
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Vitamin A Review. J. Nutr. 2016, 146, 1816S–1848S. [Google Scholar] [CrossRef] [Green Version]
- Craig, W.J.; Mangels, A.R.; American Dietetic, A. Position of the American Dietetic Association: Vegetarian diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar] [CrossRef]
- Leitzmann, C. Vegetarian diets: What are the advantages? Forum Nutr. 2005, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hever, J. Plant-Based Diets: A Physician’s Guide. Perm. J. 2016, 20, 15–82. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F.; Gardner, C.D. Dietary Protein and Amino Acids in Vegetarian Diets—A Review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef] [Green Version]
- Sakkas, H.; Bozidis, P.; Touzios, C.; Kolios, D.; Athanasiou, G.; Athanasopoulou, E.; Gerou, I.; Gartzonika, C. Nutritional Status and the Influence of the Vegan Diet on the Gut Microbiota and Human Health. Medicina 2020, 56, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobiecki, J.G.; Appleby, P.N.; Bradbury, K.E.; Key, T.J. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: Results from the European Prospective Investigation into Cancer and Nutrition-Oxford study. Nutr. Res. 2016, 36, 464–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, N.S.; Jaceldo-Siegl, K.; Sabate, J.; Fraser, G.E. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J. Acad. Nutr. Diet 2013, 113, 1610–1619. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, N.B.; Madsen, M.L.; Hansen, T.H.; Allin, K.H.; Hoppe, C.; Fagt, S.; Lausten, M.S.; Gobel, R.J.; Vestergaard, H.; Hansen, T.; et al. Intake of macro- and micronutrients in Danish vegans. Nutr. J. 2015, 14, 115. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available online: https://www.dietaryguidelines.gov (accessed on 12 January 2021).
- Alles, B.; Baudry, J.; Mejean, C.; Touvier, M.; Peneau, S.; Hercberg, S.; Kesse-Guyot, E. Comparison of Sociodemographic and Nutritional Characteristics between Self-Reported Vegetarians, Vegans, and Meat-Eaters from the NutriNet-Sante Study. Nutrients 2017, 9, 1023. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.; Ellis, F.R.; Dickerson, J.W. Studies of vegans: The fatty acid composition of plasma choline phosphoglycerides, erythrocytes, adipose tissue, and breast milk, and some indicators of susceptibility to ischemic heart disease in vegans and omnivore controls. Am. J. Clin. Nutr. 1978, 31, 805–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, T.A. Essential fatty acid requirements of vegetarians in pregnancy, lactation, and infancy. Am. J. Clin. Nutr. 1999, 70, 555S–559S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns-Whitmore, B.; Froyen, E.; Heskey, C.; Parker, T.; San Pablo, G. Alpha-Linolenic and Linoleic Fatty Acids in the Vegan Diet: Do They Require Dietary Reference Intake/Adequate Intake Special Consideration? Nutrients 2019, 11, 2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, N.; Pirotta, Y.; O’Connell, S.; Li, D.; Kelly, F.; Sinclair, A. Fatty acid composition of habitual omnivore and vegetarian diets. Lipids 2006, 41, 637–646. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Wilson Jones, G.; De Lorenzo, A.; Di Daniele, N. Potential Cardiovascular and Metabolic Beneficial Effects of omega-3 PUFA in Male Obesity Secondary Hypogonadism Syndrome. Nutrients 2020, 12, 2519. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Allen, L.H.; Bjorke-Monsen, A.L.; Brito, A.; Gueant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, F.; Samman, S. Vitamin B12 in health and disease. Nutrients 2010, 2, 299–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, W.; Schorr, H.; Obeid, R.; Geisel, J. Vitamin B-12 status, particularly holotranscobalamin II and methylmalonic acid concentrations, and hyperhomocysteinemia in vegetarians. Am. J. Clin. Nutr. 2003, 78, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, G.; Lagana, A.S.; Rapisarda, A.M.; La Ferrera, G.M.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef] [Green Version]
- Woo, K.S.; Kwok, T.C.; Celermajer, D.S. Vegan diet, subnormal vitamin B-12 status and cardiovascular health. Nutrients 2014, 6, 3259–3273. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.H. Causes of vitamin B12 and folate deficiency. Food Nutr. Bull. 2008, 29, S20–S34, discussion S35–S37. [Google Scholar] [CrossRef] [Green Version]
- Madry, E.; Lisowska, A.; Grebowiec, P.; Walkowiak, J. The impact of vegan diet on B-12 status in healthy omnivores: Five-year prospective study. Acta Sci. Pol. Technol. Aliment. 2012, 11, 209–212. [Google Scholar]
- Weikert, C.; Trefflich, I.; Menzel, J.; Obeid, R.; Longree, A.; Dierkes, J.; Meyer, K.; Herter-Aeberli, I.; Mai, K.; Stangl, G.I.; et al. Vitamin and Mineral Status in a Vegan Diet. Dtsch. Arztebl. Int. 2020, 117, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Baik, H.W.; Russell, R.M. Vitamin B12 deficiency in the elderly. Annu. Rev. Nutr. 1999, 19, 357–377. [Google Scholar] [CrossRef]
- Paul, C.; Brady, D.M. Comparative Bioavailability and Utilization of Particular Forms of B12 Supplements with Potential to Mitigate B12-related Genetic Polymorphisms. Integr. Med. 2017, 16, 42–49. [Google Scholar]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [Green Version]
- Davey, G.K.; Spencer, E.A.; Appleby, P.N.; Allen, N.E.; Knox, K.H.; Key, T.J. EPIC-Oxford: Lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003, 6, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.H.; Madsen, M.T.B.; Jorgensen, N.R.; Cohen, A.S.; Hansen, T.; Vestergaard, H.; Pedersen, O.; Allin, K.H. Bone turnover, calcium homeostasis, and vitamin D status in Danish vegans. Eur. J. Clin. Nutr. 2018, 72, 1046–1054. [Google Scholar] [CrossRef]
- Zhao, Y.; Martin, B.R.; Weaver, C.M. Calcium bioavailability of calcium carbonate fortified soymilk is equivalent to cow’s milk in young women. J. Nutr. 2005, 135, 2379–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Key, T.J.; Appleby, P.N.; Rosell, M.S. Health effects of vegetarian and vegan diets. Proc. Nutr. Soc. 2006, 65, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldmann, A.; Koschizke, J.W.; Leitzmann, C.; Hahn, A. Dietary iron intake and iron status of German female vegans: Results of the German vegan study. Ann. Nutr. Metab. 2004, 48, 103–108. [Google Scholar] [CrossRef]
- Pawlak, R.; Berger, J.; Hines, I. Iron Status of Vegetarian Adults: A Review of Literature. Am. J. Lifestyle Med. 2018, 12, 486–498. [Google Scholar] [CrossRef]
- Hurrell, R. Linking the bioavailability of iron compounds to the efficacy of iron-fortified foods. Int. J. Vitam. Nutr. Res. 2007, 77, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef] [PubMed]
- Nebl, J.; Schuchardt, J.P.; Wasserfurth, P.; Haufe, S.; Eigendorf, J.; Tegtbur, U.; Hahn, A. Characterization, dietary habits and nutritional intake of omnivorous, lacto-ovo vegetarian and vegan runners—A pilot study. BMC Nutr. 2019, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahad, F.; Ganie, S.A. Iodine, Iodine metabolism and Iodine deficiency disorders revisited. Indian J. Endocrinol. Metab. 2010, 14, 13–17. [Google Scholar]
- Zimmermann, M.B. Iodine deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef] [Green Version]
- Remer, T.; Neubert, A.; Manz, F. Increased risk of iodine deficiency with vegetarian nutrition. Br. J. Nutr. 1999, 81, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Elorinne, A.L.; Alfthan, G.; Erlund, I.; Kivimaki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and Nutrient Intake and Nutritional Status of Finnish Vegans and Non-Vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef] [Green Version]
- Tonacchera, M.; Dimida, A.; De Servi, M.; Frigeri, M.; Ferrarini, E.; De Marco, G.; Grasso, L.; Agretti, P.; Piaggi, P.; Aghini-Lombardi, F.; et al. Iodine fortification of vegetables improves human iodine nutrition: In vivo evidence for a new model of iodine prophylaxis. J. Clin. Endocrinol. Metab. 2013, 98, E694–E697. [Google Scholar] [CrossRef]
- Key, T.J.; Fraser, G.E.; Thorogood, M.; Appleby, P.N.; Beral, V.; Reeves, G.; Burr, M.L.; Chang-Claude, J.; Frentzel-Beyme, R.; Kuzma, J.W.; et al. Mortality in vegetarians and nonvegetarians: Detailed findings from a collaborative analysis of 5 prospective studies. Am. J. Clin. Nutr. 1999, 70, 516S–524S. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Umar, S.; Myint, P.K.; Mamas, M.A.; Loke, Y.K. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: A systematic review and meta-analysis. Int. J. Cardiol. 2014, 176, 680–686. [Google Scholar] [CrossRef]
- Gallu, M.; Marrone, G.; Legramante, J.M.; De Lorenzo, A.; Di Daniele, N.; Noce, A. Female Sex as a Thromboembolic Risk Factor in the Era of Nonvitamin K Antagonist Oral Anticoagulants. Cardiovasc. Ther. 2020, 2020, 1743927. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Meyer, T.E.; Klein, S.; Holloszy, J.O. Long-term low-calorie low-protein vegan diet and endurance exercise are associated with low cardiometabolic risk. Rejuvenation Res. 2007, 10, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef]
- Hervik, A.K.; Svihus, B. The Role of Fiber in Energy Balance. J. Nutr. Metab 2019, 2019, 4983657. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.H.; Kim, J.A.; Lee, J.Y. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur. J. Pharmacol. 2016, 785, 24–35. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.A. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat. Rev. Drug Discov. 2006, 5, 549–563. [Google Scholar] [CrossRef]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef]
- Petersson, H.; Basu, S.; Cederholm, T.; Riserus, U. Serum fatty acid composition and indices of stearoyl-CoA desaturase activity are associated with systemic inflammation: Longitudinal analyses in middle-aged men. Br. J. Nutr. 2008, 99, 1186–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W.C. The role of dietary n-6 fatty acids in the prevention of cardiovascular disease. J. Cardiovasc. Med. 2007, 8 (Suppl. S1), S42–S45. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Cleeman, J.I.; Merz, C.N.; Brewer, H.B., Jr.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith, S.C., Jr.; Stone, N.J.; National Heart, L.; et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004, 110, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G.F.; Steiner, G. Acute effects of insulin in the control of VLDL production in humans. Implications for the insulin-resistant state. Diabetes Care 1996, 19, 390–393. [Google Scholar] [CrossRef]
- Wallace, A.M.; McMahon, A.D.; Packard, C.J.; Kelly, A.; Shepherd, J.; Gaw, A.; Sattar, N. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation 2001, 104, 3052–3056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano-Ibanez, N.; Gea, A.; Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Corella, D.; Zomeno, M.D.; Romaguera, D.; Vioque, J.; Aros, F.; Warnberg, J.; et al. Dietary Diversity and Nutritional Adequacy among an Older Spanish Population with Metabolic Syndrome in the PREDIMED-Plus Study: A Cross-Sectional Analysis. Nutrients 2019, 11, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, J.W.; Minaker, K.L.; Pallotta, J.A.; Flier, J.S. Characterization of the insulin resistance of aging. J. Clin. Invest. 1983, 71, 1581–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisan, M.; Browne, R.; Ram, M.; Muti, P.; Freudenheim, J.; Carosella, A.M.; Armstrong, D. Correlates of markers of oxidative status in the general population. Am. J. Epidemiol. 2001, 154, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Zhu, S.; Palaniappan, L.; Heshka, S.; Carnethon, M.R.; Heymsfield, S.B. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch. Intern. Med. 2003, 163, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Riccardi, G.; Giacco, R.; Rivellese, A.A. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin. Nutr. 2004, 23, 447–456. [Google Scholar] [CrossRef]
- Vessby, B. Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Curr. Opin. Lipidol. 2003, 14, 15–19. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Gloede, L.; Green, A.A. Changes in nutrient intake and dietary quality among participants with type 2 diabetes following a low-fat vegan diet or a conventional diabetes diet for 22 weeks. J. Am. Diet. Assoc. 2008, 108, 1636–1645. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Barnard, N.D.; Scialli, A.R.; Lanou, A.J. Effects of a low-fat vegan diet and a Step II diet on macro- and micronutrient intakes in overweight postmenopausal women. Nutrition 2004, 20, 738–746. [Google Scholar] [CrossRef]
- Krauss, R.M.; Eckel, R.H.; Howard, B.; Appel, L.J.; Daniels, S.R.; Deckelbaum, R.J.; Erdman, J.W., Jr.; Kris-Etherton, P.; Goldberg, I.J.; Kotchen, T.A.; et al. AHA Dietary Guidelines: Revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000, 102, 2284–2299. [Google Scholar] [CrossRef] [Green Version]
- Parker, L.; Burns, A.C.; Sanchez, E. (Eds.) Local Government Actions to Prevent Childhood Obesity; National Academies Press: Washington, DC, USA, 2009. [Google Scholar] [CrossRef]
- Carlson, J.J.; Eisenmann, J.C.; Norman, G.J.; Ortiz, K.A.; Young, P.C. Dietary fiber and nutrient density are inversely associated with the metabolic syndrome in US adolescents. J. Am. Diet. Assoc. 2011, 111, 1688–1695. [Google Scholar] [CrossRef]
- Galisteo, M.; Duarte, J.; Zarzuelo, A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J. Nutr. Biochem. 2008, 19, 71–84. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 84, 1489–1497. [Google Scholar] [CrossRef]
- Yoo, S.; Nicklas, T.; Baranowski, T.; Zakeri, I.F.; Yang, S.J.; Srinivasan, S.R.; Berenson, G.S. Comparison of dietary intakes associated with metabolic syndrome risk factors in young adults: The Bogalusa Heart Study. Am. J. Clin. Nutr. 2004, 80, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.; Levin, S.; Trapp, C. Meat consumption as a risk factor for type 2 diabetes. Nutrients 2014, 6, 897–910. [Google Scholar] [CrossRef] [Green Version]
- Azadbakht, L.; Esmaillzadeh, A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J. Nutr. 2009, 139, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Babio, N.; Sorli, M.; Bullo, M.; Basora, J.; Ibarrola-Jurado, N.; Fernandez-Ballart, J.; Martinez-Gonzalez, M.A.; Serra-Majem, L.; Gonzalez-Perez, R.; Salas-Salvado, J.; et al. Association between red meat consumption and metabolic syndrome in a Mediterranean population at high cardiovascular risk: Cross-sectional and 1-year follow-up assessment. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.K.; Lin, Y.L.; Chen, C.L.; Ouyang, C.M.; Wu, Y.T.; Chi, Y.C.; Huang, K.C.; Yang, W.S. Reduced risk for metabolic syndrome and insulin resistance associated with ovo-lacto-vegetarian behavior in female Buddhists: A case-control study. PLoS ONE 2013, 8, e71799. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, N.S.; Sabate, J.; Jaceldo-Siegl, K.; Fraser, G.E. Vegetarian dietary patterns are associated with a lower risk of metabolic syndrome: The adventist health study 2. Diabetes Care 2011, 34, 1225–1227. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Otto, M.C.; Alonso, A.; Lee, D.H.; Delclos, G.L.; Bertoni, A.G.; Jiang, R.; Lima, J.A.; Symanski, E.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J. Nutr. 2012, 142, 526–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.H.; Linton, J.A.; Koh, S.B.; Kang, H.T. Serum ferritin concentrations predict incidence of metabolic syndrome in rural Korean adults. Clin. Chem. Lab. Med. 2012, 50, 2057–2059. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.; Harris, M. Key elements of plant-based diets associated with reduced risk of metabolic syndrome. Curr. Diabetes Rep. 2014, 14, 524. [Google Scholar] [CrossRef]
- Barnard, N.D.; Levin, S.M.; Yokoyama, Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J. Acad. Nutr. Diet 2015, 115, 954–969. [Google Scholar] [CrossRef]
- Barnard, N.D.; Scialli, A.R.; Turner-McGrievy, G.; Lanou, A.J.; Glass, J. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am. J. Med. 2005, 118, 991–997. [Google Scholar] [CrossRef]
- Huang, R.Y.; Huang, C.C.; Hu, F.B.; Chavarro, J.E. Vegetarian Diets and Weight Reduction: A Meta-Analysis of Randomized Controlled Trials. J. Gen. Intern. Med. 2016, 31, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Turner-McGrievy, G.M.; Davidson, C.R.; Wingard, E.E.; Wilcox, S.; Frongillo, E.A. Comparative effectiveness of plant-based diets for weight loss: A randomized controlled trial of five different diets. Nutrition 2015, 31, 350–358. [Google Scholar] [CrossRef]
- Moore, W.J.; McGrievy, M.E.; Turner-McGrievy, G.M. Dietary adherence and acceptability of five different diets, including vegan and vegetarian diets, for weight loss: The New DIETs study. Eat. Behav. 2015, 19, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Turner-McGrievy, G.M.; Barnard, N.D.; Scialli, A.R. A two-year randomized weight loss trial comparing a vegan diet to a more moderate low-fat diet. Obesity 2007, 15, 2276–2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Schoufour, J.D.; Rivadeneira, F.; Lamballais, S.; Ikram, M.A.; Franco, O.H.; Voortman, T. Plant-based Diet and Adiposity Over Time in a Middle-aged and Elderly Population: The Rotterdam Study. Epidemiology 2019, 30, 303–310. [Google Scholar] [CrossRef]
- Holscher, H.D.; Caporaso, J.G.; Hooda, S.; Brulc, J.M.; Fahey, G.C., Jr.; Swanson, K.S. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: Follow-up of a randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Watzl, B.; Kulling, S.E.; Moseneder, J.; Barth, S.W.; Bub, A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am. J. Clin. Nutr. 2005, 82, 1052–1058. [Google Scholar] [CrossRef]
- Dessi, M.; Noce, A.; Agnoli, A.; De Angelis, S.; Fuiano, L.; Tozzo, C.; Taccone-Gallucci, M.; Fuiano, G.; Federici, G. The usefulness of the prognostic inflammatory and nutritional index (PINI) in a haemodialysis population. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 811–815. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocedi, A.; Noce, A.; Rovella, V.; Marrone, G.; Cattani, G.; Iappelli, M.; De Paolis, P.; Iaria, G.; Sforza, D.; Gallu, M.; et al. Erythrocyte glutathione transferase in kidney transplantation: A probe for kidney detoxification efficiency. Cell Death Dis. 2018, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Bocedi, A.; Noce, A.; Marrone, G.; Noce, G.; Cattani, G.; Gambardella, G.; Di Lauro, M.; Di Daniele, N.; Ricci, G. Glutathione Transferase P1-1 an Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution. Nutrients 2019, 11, 1741. [Google Scholar] [CrossRef] [Green Version]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [Green Version]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cifkova, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campia, U.; Tesauro, M.; Di Daniele, N.; Cardillo, C. The vascular endothelin system in obesity and type 2 diabetes: Pathophysiology and therapeutic implications. Life Sci. 2014, 118, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Muralitharan, R.R.; Marques, F.Z. Diet-related gut microbial metabolites and sensing in hypertension. J. Hum. Hypertens. 2020. [Google Scholar] [CrossRef] [PubMed]
- Annalisa, N.; Alessio, T.; Claudette, T.D.; Erald, V.; Antonino, D.L.; Nicola, D.D. Gut Microbioma Population: An Indicator Really Sensible to Any Change in Age, Diet, Metabolic Syndrome, and Life-Style. Mediat. Inflamm. 2014, 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Chaimani, A.; Schwedhelm, C.; Toledo, E.; Punsch, M.; Hoffmann, G.; Boeing, H. Comparative effects of different dietary approaches on blood pressure in hypertensive and pre-hypertensive patients: A systematic review and network meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2674–2687. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian diets and blood pressure: A meta-analysis. JAMA Intern. Med. 2014, 174, 577–587. [Google Scholar] [CrossRef]
- O’Connor, L.E.; Kim, J.E.; Campbell, W.W. Total red meat intake of >/=0.5 servings/d does not negatively influence cardiovascular disease risk factors: A systemically searched meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 105, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Lopez, P.D.; Cativo, E.H.; Atlas, S.A.; Rosendorff, C. The Effect of Vegan Diets on Blood Pressure in Adults: A Meta-Analysis of Randomized Controlled Trials. Am. J. Med. 2019, 132, 875–883.e7. [Google Scholar] [CrossRef]
- Ndanuko, R.N.; Tapsell, L.C.; Charlton, K.E.; Neale, E.P.; Batterham, M.J. Dietary Patterns and Blood Pressure in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2016, 7, 76–89. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, J.; Yang, B.; Jiang, J.; Fu, Y.; Li, D. Effects of Vegetarian Diets on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015, 4, e002408. [Google Scholar] [CrossRef] [Green Version]
- Jakse, B.; Jakse, B.; Pajek, J.; Pajek, M. Effects of ad libitum consumed, low-fat, high-fiber plant-based diet supplemented with plant-based meal replacements on cardiovascular risk factors. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Mensink, R.P.; Katan, M.B. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler. Thromb. 1992, 12, 911–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Bogensberger, B.; Bencic, A.; Knuppel, S.; Boeing, H.; Hoffmann, G. Effects of oils and solid fats on blood lipids: A systematic review and network meta-analysis. J. Lipid Res. 2018, 59, 1771–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessi, M.; Noce, A.; Bertucci, P.; Noce, G.; Rizza, S.; De Stefano, A.; di Villahermosa, S.M.; Bernardini, S.; De Lorenzo, A.; Di Daniele, N. Plasma and erythrocyte membrane phospholipids and fatty acids in Italian general population and hemodialysis patients. Lipids Health Dis. 2014, 13, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Poon, S.; Seeman, E.; Hare, D.L.; Bui, M.; Iuliano, S. Fat from dairy foods and ‘meat’ consumed within recommended levels is associated with favourable serum cholesterol levels in institutionalised older adults. J. Nutr. Sci. 2019, 8, e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergeer, M.; Holleboom, A.G.; Kastelein, J.J.; Kuivenhoven, J.A. The HDL hypothesis: Does high-density lipoprotein protect from atherosclerosis? J. Lipid Res. 2010, 51, 2058–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trautwein, E.A.; Vermeer, M.A.; Hiemstra, H.; Ras, R.T. LDL-Cholesterol Lowering of Plant Sterols and Stanols-Which Factors Influence Their Efficacy? Nutrients 2018, 10, 1262. [Google Scholar] [CrossRef] [Green Version]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegard, L.; Jessup, W.; Jones, P.J.; Lutjohann, D.; Maerz, W.; Masana, L.; et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Melby, C.L.; Toohey, M.L.; Cebrick, J. Blood pressure and blood lipids among vegetarian, semivegetarian, and nonvegetarian African Americans. Am. J. Clin. Nutr. 1994, 59, 103–109. [Google Scholar] [CrossRef]
- Harman, S.K.; Parnell, W.R. The nutritional health of New Zealand vegetarian and non-vegetarian Seventh-day Adventists: Selected vitamin, mineral and lipid levels. N. Z. Med. J. 1998, 111, 91–94. [Google Scholar] [PubMed]
- Barnard, N.D.; Scialli, A.R.; Bertron, P.; Hurlock, D.; Edmonds, K.; Talev, L. Effectiveness of a low-fat vegetarian diet in altering serum lipids in healthy premenopausal women. Am. J. Cardiol. 2000, 85, 969–972. [Google Scholar] [CrossRef]
- Key, T.J.; Davey, G.K.; Appleby, P.N. Health benefits of a vegetarian diet. Proc. Nutr. Soc. 1999, 58, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Fraser, G.E. Vegetarian diets: What do we know of their effects on common chronic diseases? Am. J. Clin. Nutr. 2009, 89, 1607S–1612S. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.W.; Lin, Y.L.; Lin, T.K.; Lin, C.T.; Chen, B.C.; Lin, C.L. Total cardiovascular risk profile of Taiwanese vegetarians. Eur. J. Clin. Nutr. 2008, 62, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Chelchowska, M.; Laskowska-Klita, T.; Klemarczyk, W. Lipids and vitamin A and E status in vegetarian children. Med. Wieku Rozwoj. 2003, 7, 577–585. [Google Scholar] [PubMed]
- Klementova, M.; Thieme, L.; Haluzik, M.; Pavlovicova, R.; Hill, M.; Pelikanova, T.; Kahleova, H. A Plant-Based Meal Increases Gastrointestinal Hormones and Satiety More Than an Energy- and Macronutrient-Matched Processed-Meat Meal in T2D, Obese, and Healthy Men: A Three-Group Randomized Crossover Study. Nutrients 2019, 11, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakse, B.; Jakse, B.; Pinter, S.; Jug, B.; Godnov, U.; Pajek, J.; Fidler Mis, N. Dietary Intakes and Cardiovascular Health of Healthy Adults in Short-, Medium-, and Long-Term Whole-Food Plant-Based Lifestyle Program. Nutrients 2019, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, Y.; Levin, S.M.; Barnard, N.D. Association between plant-based diets and plasma lipids: A systematic review and meta-analysis. Nutr. Rev. 2017, 75, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.K.; Fidahusain, M.; Campbell Ii, T.M. Evaluation of an Eight-Week Whole-Food Plant-Based Lifestyle Modification Program. Nutrients 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinagre, J.C.; Vinagre, C.G.; Pozzi, F.S.; Slywitch, E.; Maranhao, R.C. Metabolism of triglyceride-rich lipoproteins and transfer of lipids to high-density lipoproteins (HDL) in vegan and omnivore subjects. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 61–67. [Google Scholar] [CrossRef]
- Karpe, F.; Steiner, G.; Uffelman, K.; Olivecrona, T.; Hamsten, A. Postprandial lipoproteins and progression of coronary atherosclerosis. Atherosclerosis 1994, 106, 83–97. [Google Scholar] [CrossRef]
- Barnard, N.D.; Goldman, D.M.; Loomis, J.F.; Kahleova, H.; Levin, S.M.; Neabore, S.; Batts, T.C. Plant-Based Diets for Cardiovascular Safety and Performance in Endurance Sports. Nutrients 2019, 11, 130. [Google Scholar] [CrossRef] [Green Version]
- Benatar, J.R.; Stewart, R.A.H. Cardiometabolic risk factors in vegans; A meta-analysis of observational studies. PLoS ONE 2018, 13, e0209086. [Google Scholar] [CrossRef] [Green Version]
- Draper, C.F.; Vassallo, I.; Di Cara, A.; Milone, C.; Comminetti, O.; Monnard, I.; Godin, J.P.; Scherer, M.; Su, M.; Jia, W.; et al. A 48-Hour Vegan Diet Challenge in Healthy Women and Men Induces a BRANCH-Chain Amino Acid Related, Health Associated, Metabolic Signature. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Toumpanakis, A.; Turnbull, T.; Alba-Barba, I. Effectiveness of plant-based diets in promoting well-being in the management of type 2 diabetes: A systematic review. BMJ Open Diabetes Res Care 2018, 6, e000534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olfert, M.D.; Wattick, R.A. Vegetarian Diets and the Risk of Diabetes. Curr. Diabetes Rep. 2018, 18, 101. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Jenkins, A.L.; Augustin, L.S.; Ludwig, D.S.; Barnard, N.D.; Anderson, J.W. Type 2 diabetes and the vegetarian diet. Am. J. Clin. Nutr. 2003, 78, 610S–616S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J.; Mayer-Davis, E.J.; Neumiller, J.J.; Nwankwo, R.; Verdi, C.L.; Urbanski, P.; et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013, 36, 3821–3842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, M. Diabetes mellitus type 2 and functional foods of plant origin. Recent Pat. Biotechnol. 2014, 8, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zuurmond, M.G.; van der Schaft, N.; Nano, J.; Wijnhoven, H.A.H.; Ikram, M.A.; Franco, O.H.; Voortman, T. Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: The Rotterdam Study. Eur. J. Epidemiol. 2018, 33, 883–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, T.H.T.; Pan, W.H.; Lin, M.N.; Lin, C.L. Vegetarian diet, change in dietary patterns, and diabetes risk: A prospective study. Nutr. Diabetes 2018, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Jaster, B.; Seidl, K.; Green, A.A.; Talpers, S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006, 29, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Rosell, M.; Appleby, P.; Spencer, E.; Key, T. Weight gain over 5 years in 21,966 meat-eating, fish-eating, vegetarian, and vegan men and women in EPIC-Oxford. Int. J. Obes. 2006, 30, 1389–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, S.; Millett, C.J.; Dhillon, P.K.; Subramanian, S.V.; Ebrahim, S. Type of vegetarian diet, obesity and diabetes in adult Indian population. Nutr. J. 2014, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonstad, S.; Butler, T.; Yan, R.; Fraser, G.E. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 2009, 32, 791–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, J.L.; Martini, M.C.; Jacobs, D.R., Jr.; Marquart, L. Plausible mechanisms for the protectiveness of whole grains. Am. J. Clin. Nutr. 1999, 70, 459S–463S. [Google Scholar] [CrossRef] [PubMed]






| Dietary Approach | Model | Characteristics of Dietary Patterns |
|---|---|---|
| PBD | VEGAN | Does not contain any animal products (meat, fish, poultry, eggs, or dairy products) but emphasizes plant-based foods, like fruit, vegetables, whole grains, legumes/beans, nuts and seeds. |
| LOV | Plant based foods, such as fruit, vegetables, whole grains, legumes/beans, cheese and various dairy products. | |
| FV | Plant-based foods, such as fruit, vegetables, whole grains, legumes/beans, fish and seafoods. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrone, G.; Guerriero, C.; Palazzetti, D.; Lido, P.; Marolla, A.; Di Daniele, F.; Noce, A. Vegan Diet Health Benefits in Metabolic Syndrome. Nutrients 2021, 13, 817. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030817
Marrone G, Guerriero C, Palazzetti D, Lido P, Marolla A, Di Daniele F, Noce A. Vegan Diet Health Benefits in Metabolic Syndrome. Nutrients. 2021; 13(3):817. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030817
Chicago/Turabian StyleMarrone, Giulia, Cristina Guerriero, Daniela Palazzetti, Paolo Lido, Alessandro Marolla, Francesca Di Daniele, and Annalisa Noce. 2021. "Vegan Diet Health Benefits in Metabolic Syndrome" Nutrients 13, no. 3: 817. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13030817