Magnesium Influences Membrane Fusion during Myogenesis by Modulating Oxidative Stress in C2C12 Myoblasts
Abstract
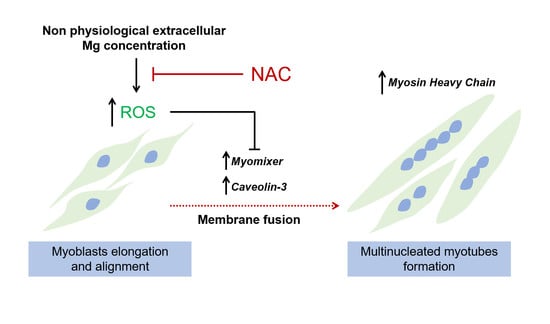
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. SDS-PAGE and Western Blot
2.3. Immunofluorescence
2.4. ROS Production Analysis
2.5. Statistical Analysis
3. Results
3.1. Low and High Extracellular Mg Impair Myogenesis
3.2. Low and High Mg Induce ROS Accumulation during Myogenesis
3.3. ROS Accumulation Is Involved in the Impairment of Myogenesis
3.4. Low and High Mg Impair Myoblasts Fusion
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Welch, A.A.; Kelaiditi, E.; Jennings, A.; Steves, C.J.; Spector, T.D.; MacGregor, A. Dietary Magnesium Is Positively Associated with Skeletal Muscle Power and Indices of Muscle Mass and May Attenuate the Association between Circulating C-Reactive Protein and Muscle Mass in Women. J. Bone Miner. Res. 2016, 31, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Welch, A.A.; Skinner, J.; Hickson, M. Dietary magnesium may be protective for aging of bone and skeletal muscle in middle and younger older age men and women: Cross-sectional findings from the UK biobank cohort. Nutrients 2017, 9, 1189. [Google Scholar] [CrossRef] [Green Version]
- Knochel, J.P.; Cronin, R.E. The myopathy of experimental magnesium deficiency. Adv. Exp. Med. Biol. 1984, 178, 351–361. [Google Scholar] [CrossRef]
- Molkentin, J.D.; Olson, E.N. Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 1996, 6, 445–453. [Google Scholar] [CrossRef]
- Berkes, C.A.; Tapscott, S.J. MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 2005, 16, 585–595. [Google Scholar] [CrossRef]
- Taylor, M.V.; Hughes, S.M. Mef2 and the skeletal muscle differentiation program. Semin. Cell Dev. Biol. 2017, 72, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinska, D.; Kudin, A.P.; Bejtka, M.; Kunz, W.S. Changes in mitochondrial reactive oxygen species synthesis during differentiation of skeletal muscle cells. Mitochondrion 2012, 12, 144–148. [Google Scholar] [CrossRef]
- Andrés, V.; Walsh, K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J. Cell Biol. 1996, 132, 657–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burattini, S.; Ferri, R.; Battistelli, M.; Curci, R.; Luchetti, F.; Falcieri, E. C2C12 murine myoblasts as a model of skeletal muscle development: Morpho-functional characterization. Eur. J. Histochem. 2004, 48, 223–233. [Google Scholar] [CrossRef]
- Cazzaniga, A.; Ille, F.; Wuest, S.; Haack, C.; Koller, A.; Giger-Lange, C.; Zocchi, M.; Egli, M.; Castiglioni, S.; Maier, J.A. Scalable microgravity simulator used for long-term musculoskeletal cells and tissue engineering. Int. J. Mol. Sci. 2020, 21, 8908. [Google Scholar] [CrossRef]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.M.; Grande, A.; Frassineti, C. Magnesium is a key regulator of the balance between osteoclast and osteoblast differentiation in the presence of vitamin D 3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galán, I.; Vega, A.; Goicoechea, M.; Shabaka, A.; Gatius, S.; Abad, S.; López-gómez, J.M. Hypermagnesemia is Associated with All-Cause Mortality in Patients with Chronic Kidney Disease. J. Clin. Exp. Nephrol. 2020, 1–8. [Google Scholar] [CrossRef]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef] [Green Version]
- Rajasekaran, N.S.; Shelar, S.B.; Jones, D.P.; Hoidal, J.R. Reductive stress impairs myogenic differentiation. Redox Biol. 2020, 34, 101492. [Google Scholar] [CrossRef]
- Rochard, P.; Rodier, A.; Casas, F.; Cassar-Malek, I.; Marchal-Victorion, S.; Daury, L.; Wrutniak, C.; Cabello, G. Mitochondrial activity is involved in the regulation of myoblast differentiation through myogenin expression and activity of myogenic factors. J. Biol. Chem. 2000, 275, 2733–2744. [Google Scholar] [CrossRef] [Green Version]
- Ardite, E.; Barbera, J.A.; Roca, J.; Fernández-Checa, J.C. Glutathione depletion impairs myogenic differentiation of murine skeletal muscle C2C12 cells through sustained NF-kappaB activation. Am. J. Pathol. 2004, 165, 719–728. [Google Scholar] [CrossRef]
- Sargenti, A.; Castiglioni, S.; Olivi, E.; Bianchi, F.; Cazzaniga, A.; Farruggia, G.; Cappadone, C.; Merolle, L.; Malucelli, E.; Ventura, C.; et al. Magnesium deprivation potentiates human mesenchymal stem cell transcriptional remodeling. Int. J. Mol. Sci. 2018, 19, 1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locatelli, L.; Fedele, G.; Castiglioni, S.; Maier, J.A. Magnesium deficiency induces lipid accumulation in vascular endothelial cells via oxidative stress—the potential contribution of edf-1 and pparγ. Int. J. Mol. Sci. 2021, 22, 1050. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, F.; Volonté, D.; Engelman, J.A.; Scherer, P.E.; Lisanti, M.P. Targeted down-regulation of caveolin-3 is sufficient to inhibit myotube formation in differentiating C2C12 myoblasts. Transient activation of p38 mitogen-activated protein kinase is required for induction of caveolin-3 expression and subsequent myotube fo. J. Biol. Chem. 1999, 274, 30315–30321. [Google Scholar] [CrossRef] [Green Version]
- Volonte, D.; Peoples, A.J.; Galbiati, F. Modulation of Myoblast Fusion by Caveolin-3 in Dystrophic Skeletal Muscle Cells: Implications for Duchenne Muscular Dystrophy and Limb-Girdle Muscular Dystrophy-1C. Mol. Biol. Cell 2003, 14, 4075–4088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, P.; Ramirez-Martinez, A.; Li, H.; Cannavino, J.; McAnally, J.R.; Shelton, J.M.; Sánchez-Ortiz, E.; Bassel-Duby, R.; Olson, E.N. Control of muscle formation by the fusogenic micropeptide myomixer. Science 2017, 356, 323–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; You, W.; Wang, Y.; Shan, T. The regulatory role of Myomaker and Myomixer–Myomerger–Minion in muscle development and regeneration. Cell. Mol. Life Sci. 2020, 77, 1551–1569. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zocchi, M.; Béchet, D.; Mazur, A.; Maier, J.A.; Castiglioni, S. Magnesium Influences Membrane Fusion during Myogenesis by Modulating Oxidative Stress in C2C12 Myoblasts. Nutrients 2021, 13, 1049. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13041049
Zocchi M, Béchet D, Mazur A, Maier JA, Castiglioni S. Magnesium Influences Membrane Fusion during Myogenesis by Modulating Oxidative Stress in C2C12 Myoblasts. Nutrients. 2021; 13(4):1049. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13041049
Chicago/Turabian StyleZocchi, Monica, Daniel Béchet, André Mazur, Jeanette A. Maier, and Sara Castiglioni. 2021. "Magnesium Influences Membrane Fusion during Myogenesis by Modulating Oxidative Stress in C2C12 Myoblasts" Nutrients 13, no. 4: 1049. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13041049