Botanicals in Postmenopausal Osteoporosis
Abstract
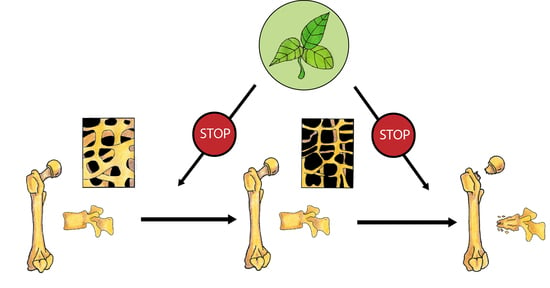
:1. Introduction
2. Phytoestrogens
2.1. Isoflavones
2.1.1. Soybean in Clinical Trials
2.1.2. Red Clover in Clinical Trials
2.1.3. Soybean and Red Clover in Animal Studies and In Vitro Models
2.1.4. Other Plants Containing Isoflavones
Alfalfa
Pueraria candollei var. mirifica
2.2. Other Plants Containing Phytoestrogens Investigated in Osteoporosis Treatment
2.2.1. Epimedium (Berberidaceae)
Epimedium in Clinical Trials
Epimedium in Animal Models and In Vitro Studies
2.2.2. Hop (Humulus lupulus L.)
3. Other Botanicals
3.1. Dried Plums
3.1.1. Dried Plums in Clinical Trials
3.1.2. Dried Plums in Animal Studies and In Vitro Models
3.2. Horsetail (Equisetum arvense)
3.3. Black Cohosh (Cimcifuga racemosa)
3.4. Salvia miltiorrhiza and Salvia plebia
3.5. Other Herbs
3.5.1. Labisia pumila and Eurycoma longifolia
3.5.2. Drynaria fortunei
3.5.3. Other Plant-Derived Constituents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| ALP | alkaline phosphatase |
| BALP | bone-specific alkaline phosphatase |
| bFGF | basic fibroblast growth factor |
| BMC | bone mineral content (BMC) |
| BMD | bone mineral density |
| BMP | bone morphogenic protein |
| BMSC | bone marrow-derived mesenchymal stem cells |
| BV/TV | bone fraction |
| c-FMS | colony-stimulating factor-1 receptor |
| Conn.D. | connectivity density |
| CTX | type I collagen crosslinked beta C-telopeptide |
| DPD | deoxypyridinoline |
| ER | oestrogen receptor |
| ERK | extracellular signal-regulated kinases |
| FasL | Fas ligand |
| bFGF | basic fibroblast growth factor |
| Hh | Hedgehog |
| HRT | hormonal replacement therapy |
| IGF | insulin-like growth factor |
| IL-6 | interleukin 6 |
| JNK | c-Jun N terminal kinase |
| MAPK | mitogen-activated protein kinase |
| M-CSF | macrophage colony-stimulating factor |
| mCT | micro-computed tomography |
| mRNA | messenger ribonucleic acid |
| mTOR | mechanistic target of rapamycin |
| NFAT-c1 | Nuclear factor of activated T-cells, cytoplasmic 1 |
| NFκB | nuclear factor-kappa B |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NTX | type I collagen crosslinked N- telopeptide |
| OB | osteoblast |
| OC | osteoclast |
| OPG | osteoprotegerin |
| OSX | Osterix |
| P1NP | type I procollagen-N-propeptide |
| PDGF | platelet-derived growth factor |
| PI3K/AKT | phosphoinositide-3-kinase/serine-threonine protein kinase B |
| PLC | phospholipase C |
| pre-OB | pre-osteoblasts |
| pre-OC | pre-osteoclasts |
| PTH | parathyroid hormone |
| RANK | Receptor Activator for Nuclear Factor κB |
| RANKL | Receptor Activator for Nuclear Factor κB Ligand |
| RCT | randomized clinical trial |
| Runx2 | Runt-related transcription factor 2 |
| SERM | Selective Oestrogen Receptor Modulator |
| sFasL | soluble Fas lignad |
| SMI | Structural Model Inde |
| Tb.N. | number of trabeculae |
| Tb.Sp. | separation of trabeculae |
| Tb.Th. | trabecular thickness |
| TGFβ | tumour growth factor β |
| TRAF6 | tumour necrosis factor receptor associated factor 6 |
| TRAP 5b | Tartrate-resistant acid phosphatase 5b |
References
- Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [CrossRef]
- Bartl, R.; Bartl, C. Epidemiology of osteoporotic fractures. In The Osteoporosis Manual; Springer International Publishing: Cham, Switzerland, 2019; pp. 231–232. [Google Scholar]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.E.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanis, J.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; On behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF); Cooper, C.; Rizzoli, R.; Reginster, J.-Y. European guidance for the diagnosis and management of osteoporosis in Postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [Green Version]
- Bellavia, D.; Dimarco, E.; Costa, V.; Carina, V.; De Luca, A.; Raimondi, L.; Fini, M.; Gentile, C.; Caradonna, F.; Giavaresi, G. Flavonoids in Bone Erosive Diseases: Perspectives in Osteoporosis Treatment. Trends Endocrinol. Metab. 2021, 32, 76–94. [Google Scholar] [CrossRef]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Khalid, A.B.; Krum, S.A. Estrogen receptors alpha and beta in bone. Bone 2016, 87, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Krum, S.A.; Chang, J.; Miranda-Carboni, G.; Wang, C.-Y. Novel functions for NFκB: Inhibition of bone formation. Nat. Rev. Rheumatol. 2010, 6, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.J.; Tom, C.; Guemes, M.; Polanco, G.; Mayorga, M.E.; Wend, K.; Miranda-Carboni, G.A.; Krum, S.A. ERα signaling regulates MMP3 expression to induce FasL cleavage and osteoclast apoptosis. J. Bone Miner. Res. 2013, 28, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Xiong, J.; Koromila, T.; Ji, J.S.; Chang, S.; Song, Y.S.; Miller, J.L.; Han, C.-Y.; Kostenuik, P.; Krum, S.A.; et al. Estrogens antagonize RUNX2-mediated osteoblast-driven osteoclastogenesis through regulating RANKL membrane association. Bone 2015, 75, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Gazzerro, E.; Canalis, E. Bone morphogenetic proteins and their antagonists. Rev. Endocr. Metab. Disord. 2006, 7, 51–65. [Google Scholar] [CrossRef]
- Canalis, E. Wnt signalling in osteoporosis: Mechanisms and novel therapeutic approaches. Nat. Rev. Endocrinol. 2013, 9, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E. Skeletal Growth Factors. In Osteoporosis; Elsevier Academic Press: Cambridge, MA, USA, 2013; pp. 391–410. [Google Scholar]
- Gómez-Zorita, S.; González-Arceo, M.; Fernández-Quintela, A.; Eseberri, I.; Trepiana, J.; Portillo, M.P. Scientific Evidence Supporting the Beneficial Effects of Isoflavones on Human Health. Nutrients 2020, 12, 3853. [Google Scholar] [CrossRef]
- Sansai, K.; Na Takuathung, M.; Khatsri, R.; Teekachunhatean, S.; Hanprasertpong, N.; Koonrungsesomboon, N. Effects of isoflavone interventions on bone mineral density in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2020, 31, 1853–1864. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Yang, D.; Zhen, W.; Zhang, J.; Peng, S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 2018, 29, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Lin, M.; Li, X.; Li, C.; Gao, B.; Gan, H.; Yang, Z.; Lin, X.; Liao, L.; Yang, M. Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line. Int. J. Mol. Med. 2012, 30, 889–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Zhao, J.; Zhang, X.; Li, H.; Zhou, Y. Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur. J. Pharmacol. 2013, 714, 15–22. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, G.; Liu, X.; Dai, M.; Zhang, B. Icariin inhibits RANKL-induced osteoclastogenesis via modulation of the NF-κB and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2019, 508, 902–906. [Google Scholar] [CrossRef]
- Ming, L.-G.; Lv, X.; Ma, X.-N.; Ge, B.-F.; Zhen, P.; Song, P.; Zhou, J.; Ma, H.-P.; Xian, C.J.; Chen, K.-M. The Prenyl Group Contributes to Activities of Phytoestrogen 8-Prenynaringenin in Enhancing Bone Formation and Inhibiting Bone Resorption In Vitro. Endocrinology 2013, 154, 1202–1214. [Google Scholar] [CrossRef] [Green Version]
- Indran, I.R.; Liang, R.L.Z.; Min, T.E.; Yong, E.-L. Preclinical studies and clinical evaluation of compounds from the genus Epimedium for osteoporosis and bone health. Pharmacol. Ther. 2016, 162, 188–205. [Google Scholar] [CrossRef]
- Kim, H.-K.; Woo, E.-R.; Lee, H.-W.; Park, H.-R.; Kim, H.-N.; Jung, Y.-K.; Choi, J.-Y.; Chae, S.-W.; Kim, H.-R.; Chae, H.-J. The Correlation of Salvia miltiorrhiza Extract–Induced Regulation of Osteoclastogenesis with the Amount of Components Tanshinone I, Tanshinone IIA, Cryptotanshinone, and Dihydrotanshinone. Immunopharmacol. Immunotoxicol. 2008, 30, 347–364. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, S.; Zhao, Y.; Sun, Y.; Xu, Z.; Yuan, B.; Chen, X. Tanshinone IIA attenuates osteoclastogenesis in ovariecto-mized mice by inactivating NF-κB and Akt signaling pathways. Am. J. Transl. Res. 2018, 10, 1457–1468. [Google Scholar]
- Cui, L.; Liu, Y.-Y.; Wu, T.; Ai, C.-M.; Chen, H.-Q. Osteogenic effects of D(+)β-3,4-dihydroxyphenyl lactic acid (salvianic acid A, SAA) on osteoblasts and bone marrow stromal cells of intact and prednisone-treated rats. Acta Pharmacol. Sin. 2009, 30, 321–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Li, T.; Liu, Y.; Zhou, L.; Li, P.; Xu, B.; Huang, L.; Chen, Y.; Liu, Y.; Tian, X.; et al. Salvianolic Acid B Prevents Bone Loss in Prednisone-Treated Rats through Stimulation of Osteogenesis and Bone Marrow Angiogenesis. PLoS ONE 2012, 7, e34647. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-H.; Lim, H.-J.; Bak, S.G.; Park, E.-J.; Jang, H.-J.; Lee, S.; Lee, S.; Lee, K.M.; Cheong, S.H.; Lee, S.-J.; et al. Eudebeiolide B Inhibits Osteoclastogenesis and Prevents Ovariectomy-Induced Bone Loss by Regulating RANKL-Induced NF-κB, c-Fos and Calcium Signaling. Pharmaceuticals 2020, 13, 468. [Google Scholar] [CrossRef]
- Scheiber, M.D.; Liu, J.H.; Subbiah, M.T.R.; Rebar, R.W.; Setchell, K.D.R. Dietary inclusion of whole soy foods results in significant reductions in clinical risk factors for osteoporosis and cardiovascular disease in normal postmenopausal women. Menopause 2001, 8, 384–392. [Google Scholar] [CrossRef]
- Chiechi, L.M.; Secreto, G.; D’Amore, M.; Fanelli, M.; Venturelli, E.; Cantatore, F.; Valerio, T.; LaSelva, G.; Loizzi, P. Efficacy of a soy rich diet in preventing postmenopausal osteoporosis: The Menfis randomized trial. Maturitas 2002, 42, 295–300. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Ho, S.C.; Lam, S.S.H.; Ho, S.S.S.; Woo, J.L.F. Beneficial effect of soy isoflavones on bone mineral content was modified by years since menopause, body weight, and calcium intake: A double-blind, randomized, controlled trial. Menopause 2004, 11, 246–254. [Google Scholar] [CrossRef]
- Ye, Y.-B.; Tang, X.-Y.; Verbruggen, M.A.; Su, Y.-X. Soy isoflavones attenuate bone loss in early postmenopausal Chinese women. Eur. J. Nutr. 2006, 45, 327–334. [Google Scholar] [CrossRef]
- Wu, J.; Oka, J.; Ezaki, J.; Ohtomo, T.; Ueno, T.; Uchiyama, S.; Toda, T.; Uehara, M.; Ishimi, Y. Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women. Menopause 2007, 14, 866–874. [Google Scholar] [CrossRef]
- Shedd-Wise, K.M.; Alekel, D.L.; Hofmann, H.; Hanson, K.B.; Schiferl, D.J.; Hanson, L.N.; Van Loan, M.D. The Soy Isoflavones for Reducing Bone Loss Study: 3-Yr Effects on pQCT Bone Mineral Density and Strength Measures in Postmenopausal Women. J. Clin. Densitom. 2011, 14, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Choue, R.; Lim, H. Effect of soy isoflavones supplement on climacteric symptoms, bone biomarkers, and quality of life in Korean postmenopausal women: A randomized clinical trial. Nutr. Res. Pract. 2017, 11, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tit, D.M.; Bungau, S.; Iovan, C.; Cseppento, D.C.N.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef] [Green Version]
- Sathyapalan, T.; Aye, M.; Rigby, A.S.; Fraser, W.D.; Thatcher, N.J.; Kilpatrick, E.S.; Atkin, S.L. Soy Reduces Bone Turnover Markers in Women During Early Menopause: A Randomized Controlled Trial. J. Bone Miner. Res. 2016, 32, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levis, S.; Strickman-Stein, N.; Ganjei-Azar, P.; Xu, P.; Doerge, D.R.; Krischer, J. Soy Isoflavones in the Prevention of Menopausal Bone Loss and Menopausal Symptoms. Arch. Intern. Med. 2011, 171, 1363–1369. [Google Scholar] [CrossRef] [Green Version]
- Kreijkamp-Kaspers, S.; Kok, L.; Grobbee, D.E.; De Haan, E.H.F.; Aleman, A.; Lampe, J.W.; Van Der Schouw, Y.T. Effect of Soy Protein Containing Isoflavones on Cognitive Function, Bone Mineral Density, and Plasma Lipids in Postmenopausal Women. JAMA 2004, 292, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brink, E.; Coxam, V.; Robins, S.; Wahala, K.; Cassidy, A.; Branca, F.; PHYTOS Investigators. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: A randomized, double-blind, placebo controlled study. Am. J. Clin. Nutr. 2008, 87, 761–770. [Google Scholar] [CrossRef]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. Effects of the Phytoestrogen Genistein on Bone Metabolism in Osteopenic Postmenopausal Women. Ann. Intern. Med. 2007, 146, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.R.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; D’Anna, R.; et al. Breast Safety and Efficacy of Genistein Aglycone for Postmenopausal Bone Loss: A Follow-Up Study. J. Clin. Endocrinol. Metab. 2008, 93, 4787–4796. [Google Scholar] [CrossRef] [Green Version]
- Atteritano, M.; Mazzaferro, S.; Frisina, A.; Cannata, M.L.; Bitto, A.; D’Anna, R.; Squadrito, F.; Macrì, I.; Frisina, N.; Buemi, M. Genistein effects on quantitative ultrasound parameters and bone mineral density in osteopenic postmenopausal women. Osteoporos. Int. 2009, 20, 1947–1954. [Google Scholar] [CrossRef]
- Lappe, J.; Kunz, I.; Bendik, I.; Prudence, K.; Weber, P.; Recker, R.; Heaney, R.P. Effect of a combination of genistein, polyunsaturated fatty acids and vitamins D3 and K1 on bone mineral density in postmenopausal women: A randomized, placebo-controlled, double-blind pilot study. Eur. J. Nutr. 2013, 52, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Arcoraci, V.; Atteritano, M.; Squadrito, F.; D’Anna, R.; Marini, H.R.; Santoro, D.; Minutoli, L.; Messina, S.; Altavilla, D.; Bitto, A. Antiosteoporotic Activity of Genistein Aglycone in Postmenopausal Women: Evidence from a Post-Hoc Analysis of a Multicenter Randomized Controlled Trial. Nutrients 2017, 9, 179. [Google Scholar] [CrossRef] [Green Version]
- Clifton-Bligh, P.B.; Baber, R.J.; Fulcher, G.R.; Nery, M.-L.; Moreton, T. The effect of isoflavones extracted from red clover (Rimostil) on lipid and bone metabolism. Menopause 2001, 8, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Compston, J.E.; Day, N.E.; Dowsett, M.; Bingham, S.A. The effects of phytoestrogen isoflavones on bone density in women: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2004, 79, 326–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, ajcn153353-920. [Google Scholar] [CrossRef] [Green Version]
- Thorup, A.C.; Lambert, M.N.; Kahr, H.S.; Bjerre, M.; Jeppesen, P.B. Intake of Novel Red Clover Supplementation for 12 Weeks Improves Bone Status in Healthy Menopausal Women. Evid.-Based Complement. Altern. Med. 2015, 2015, 9138. [Google Scholar] [CrossRef] [Green Version]
- Powles, T.J.; Howell, A.; Evans, D.G.; McCloskey, E.V.; Ashley, S.; Greenhalgh, R.; Affen, J.; Flook, L.A.; Tidy, A. Red clover isoflavones are safe and well tolerated in women with a family history of breast cancer. Menopause Int. Integr. J. Postreproductive Health 2008, 14, 6–12. [Google Scholar] [CrossRef]
- Schult, T.M.K.; Ensrud, K.E.; Blackwell, T.; Ettinger, B.; Wallace, R.; Tice, J.A. Effect of isoflavones on lipids and bone turnover markers in menopausal women. Maturitas 2004, 48, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Manonai, J.; Chittacharoen, A.; Udomsubpayakul, U.; Theppisai, H.; Theppisai, U. Effects and safety of Pueraria mirifica on lipid profiles and biochemical markers of bone turnover rates in healthy postmenopausal women. Menopause 2008, 15, 530–535. [Google Scholar] [CrossRef]
- Okamura, S.; Sawada, Y.; Satoh, T.; Sakamoto, H.; Saito, Y.; Sumino, H.; Takizawa, T.; Kogure, T.; Chaichantipyuth, C.; Higuchi, Y.; et al. Pueraria Mirifica Phytoestrogens Improve Dyslipidemia in Postmenopausal Women Probably by Activating Estrogen Receptor Subtypes. Tohoku J. Exp. Med. 2008, 216, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Qin, L.; Shi, Y. Epimedium-Derived Phytoestrogen Flavonoids Exert Beneficial Effect on Preventing Bone Loss in Late Postmenopausal Women: A 24-Month Randomized, Double-Blind and Placebo-Controlled Trial. J. Bone Miner. Res. 2007, 22, 1072–1079. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Khalil, D.A.; Lucas, E.A.; Georgis, A.; Stoecker, B.J.; Hardin, C.; Payton, M.E.; Wild, R.A. Dried Plums Improve Indices of Bone Formation in Postmenopausal Women. J. Women’s Heal. Gender-Based Med. 2002, 11, 61–68. [Google Scholar] [CrossRef]
- Hooshmand, S.; Brisco, J.R.Y.; Arjmandi, B.H. The effect of dried plum on serum levels of receptor activator of NF-κB ligand, osteoprotegerin and sclerostin in osteopenic postmenopausal women: A randomised controlled trial. Br. J. Nutr. 2014, 112, 55–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooshmand, S.; Kern, M.; Metti, D.; Shamloufard, P.; Chai, S.C.; Johnson, S.A.; Payton, M.E.; Arjmandi, B.H. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: A randomized, controlled trial. Osteoporos. Int. 2016, 27, 2271–2279. [Google Scholar] [CrossRef]
- Arjmandi, B.; George, K.; Ormsbee, L.; Akhavan, N.; Munoz, J.; Foley, E.; Siebert, S. The Short-Term Effects of Prunes in Preventing Inflammation and Improving Indices of Bone Health in Osteopenic Men. Curr. Dev. Nutr. 2020, 4, 5. [Google Scholar] [CrossRef]
- Corletto, F. Female climacteric osteoporosis therapy with titrated horsetail (Equisetum arvense) extract plus calcium (osteosil calcium): Randomized double blind study. Minerva Ortop. Traumatol. 1999, 50, 201–206. [Google Scholar]
- Wuttke, W.; Gorkow, C.; Seidlová-Wuttke, D. Effects of black cohosh (Cimicifuga racemosa) on bone turnover, vaginal mucosa, and various blood parameters in postmenopausal women. Menopause 2006, 13, 185–196. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, M.A.; Pineda, B.; Hermenegildo, C.; Tarín, J.J.; Cano, A. Isopropanolic Cimicifuga racemosa is favorable on bone markers but neutral on an osteoblastic cell line. Fertil. Steril. 2009, 91, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Bebenek, M.; Kemmler, W.; von Stengel, S.; Engelke, K.; Kalender, W.A. Effect of exercise and Cimicifuga racemosa (CR BNO 1055) on bone mineral density, 10-year coronary heart disease risk, and menopausal complaints. Menopause 2010, 17, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, S.M.; George, A.; Evans, M.; Anthony, J. Efficacy of Labisia pumila and Eurycoma longifolia standardised extracts on hot flushes, quality of life, hormone and lipid profile of peri-menopausal and menopausal women: A randomised, placebo-controlled study. Food Nutr. Res. 2020, 64, 1–15. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; Peñalvo, J.; Hollman, P. Bioavailability of isoflavones in humans. In Flavonoids and Related Compounds: Bioavail-ability and Function; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439848272. [Google Scholar]
- Jolly, J.J.; Chin, K.-Y.; Alias, E.; Chua, K.H.; Soelaiman, I.N. Protective Effects of Selected Botanical Agents on Bone. Int. J. Environ. Res. Public Health 2018, 15, 963. [Google Scholar] [CrossRef] [Green Version]
- Collison, M.W. Determination of Total Soy Isoflavones in Dietary Supplements, Supplement Ingredients, and Soy Foods by High-Performance Liquid Chromatography with Ultraviolet Detection: Collaborative Study. J. AOAC Int. 2008, 91, 489–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Shu, X.-O.; Li, H.; Yang, G.; Li, Q.; Gao, Y.-T.; Zheng, W. Prospective Cohort Study of Soy Food Consumption and Risk of Bone Fracture Among Postmenopausal Women. Arch. Intern. Med. 2005, 165, 1890–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennison, E.; Yoshimura, N.; Hashimoto, T.; Cooper, C. Bone loss in Great Britain and Japan: A comparative longitudinal study. Bone 1998, 23, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Norimatsu, H.; Davis, J.W.; Yano, K.; Wasnich, R.D.; Fujiwara, S.; Hosoda, Y.; Melton, I.L.J. A Comparison of Hip Fracture Incidence among Native Japanese, Japanese Americans, and American Caucasians. Am. J. Epidemiol. 1991, 133, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atteritano, M.; Pernice, F.; Mazzaferro, S.; Mantuano, S.; Frisina, A.; D’Anna, R.; Cannata, M.L.; Bitto, A.; Squadrito, F.; Frisina, N.; et al. Effects of phytoestrogen genistein on cytogenetic biomarkers in postmenopausal women: 1 year randomized, placebo-controlled study. Eur. J. Pharmacol. 2008, 589, 22–26. [Google Scholar] [CrossRef]
- Pawlowski, J.W.; Martin, B.R.; McCabe, G.P.; McCabe, L.D.; Jackson, G.S.; Peacock, M.; Barnes, S.; Weaver, C.M. Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: A randomized crossover trial. Am. J. Clin. Nutr. 2015, 102, 695–703. [Google Scholar] [CrossRef] [Green Version]
- Lambert, M.N.T.; Hu, L.M.; Jeppesen, P.B. A systematic review and meta-analysis of the effects of isoflavone formulations against estrogen-deficient bone resorption in peri- and postmenopausal women. Am. J. Clin. Nutr. 2017, 106, ajcn151464-811. [Google Scholar] [CrossRef] [Green Version]
- Akhlaghi, M.; Nasab, M.G.; Riasatian, M.; Sadeghi, F. Soy isoflavones prevent bone resorption and loss, a systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 60, 2327–2341. [Google Scholar] [CrossRef]
- Abdi, F.; Alimoradi, Z.; Haqi, P.; Mahdizad, F. Effects of phytoestrogens on bone mineral density during the menopause transition: A systematic review of randomized, controlled trials. Climacteric 2016, 19, 535–545. [Google Scholar] [CrossRef]
- Liu, J.; Ho, S.C.; Su, Y.-X.; Chen, W.-Q.; Zhang, C.-X.; Chen, Y.-M. Effect of long-term intervention of soy isoflavones on bone mineral density in women: A meta-analysis of randomized controlled trials. Bone 2009, 44, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-X.; Wu, P.; Mao, Y.-F.; Wang, B.; Zhang, J.-F.; Chen, W.-L.; Liu, Z.; Shi, X.-L. Chinese Herbal Medicine for Osteoporosis: A Meta-analysis of Randomized Controlled Trials. J. Clin. Densitom. 2017, 20, 516–525. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, Y.; Wang, Y.; Cui, J.; Feng, K.; Kong, X.; Chen, L. Psoralidin, a prenylated coumestan, as a novel anti-osteoporosis candidate to enhance bone formation of osteoblasts and decrease bone resorption of osteoclasts. Eur. J. Pharmacol. 2017, 801, 62–71. [Google Scholar] [CrossRef]
- Chopra, B.; Dhingra, A.K.; Dhar, K.L. Psoralea corylifolia L. (Buguchi)—Folklore to modern evidence: Review. Fitoterapia 2013, 90, 44–56. [Google Scholar] [CrossRef]
- Nieves, J.W. Skeletal effects of nutrients and nutraceuticals, beyond calcium and vitamin D. Osteoporos. Int. 2013, 24, 771–786. [Google Scholar] [CrossRef]
- Kim, M.R.; Kim, H.J.; Yu, S.H.; Lee, B.S.; Jeon, S.Y.; Lee, J.J.; Lee, Y.C. Combination of Red Clover and Hops Extract Improved Menopause Symptoms in an Ovariectomized Rat Model. Evid.-Based Complement. Altern. Med. 2020, 2020, 1391. [Google Scholar] [CrossRef]
- Geller, S.E.; Studee, L. Soy and red clover for mid-life and aging. Climacteric 2006, 9, 245–263. [Google Scholar] [CrossRef] [Green Version]
- Booth, N.L.; Piersen, C.E.; Banuvar, S.; Geller, S.E.; Shulman, L.P.; Farnsworth, N.R. Clinical studies of red clover (Trifolium pratense) dietary supplements in menopause: A literature review. Menopause 2006, 13, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Moon, H.-J.; Paik, D.-J.; Kim, D.-Y. Effect of dietary legumes on bone-specific gene expression in ovariectomized rats. Nutr. Res. Pract. 2013, 7, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, J.S.; Han, Y.S.; Lee, S.S. The Effects of Yellow Soybean, Black Soybean, and Sword Bean on Lipid Levels and Oxidative Stress in Ovariectomized Rats. Int. J. Vitam. Nutr. Res. 2010, 80, 97–106. [Google Scholar] [CrossRef]
- Hinton, P.S.; Ortinau, L.C.; Dirkes, R.K.; Shaw, E.L.; Richard, M.W.; Zidon, T.Z.; Britton, S.L.; Koch, L.G.; Vieira-Potter, V.J. Soy protein improves tibial whole-bone and tissue-level biomechanical properties in ovariectomized and ovary-intact, low-fit female rats. Bone Rep. 2018, 8, 244–254. [Google Scholar] [CrossRef]
- Akao, M.; Abe, R.; Sato, N.; Hasegawa-Tanigome, A.; Kumagai, H.; Kumagai, H. Prevention of Osteoporosis by Oral Administration of Phytate-Removed and Deamidated Soybean β-Conglycinin. Int. J. Mol. Sci. 2015, 16, 2117–2129. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.-W.; Choi, S.-W.; Kim, H.-J.; Lee, K.-S.; Kim, S.-H.; Kim, S.-L.; Do, S.H.; Seo, W.-D. Germinated soy germ with increased soyasaponin Ab improves BMP-2-induced bone formation and protects against in vivo bone loss in osteoporosis. Sci. Rep. 2018, 8, 12970. [Google Scholar] [CrossRef]
- Noh, D.; Lim, Y.; Lee, H.; Kim, H.; Kwon, O. Soybean-Hop Alleviates Estrogen Deficiency-Related Bone Loss and Metabolic Dysfunction in Ovariectomized Rats Fed a High-Fat Diet. Molecules 2018, 23, 1205. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-S.; Lee, H.; Nirmala, F.S.; Jung, C.H.; Jang, Y.-J.; Ha, T.-Y.; Ahn, J. Dry-Fermented Soybean Food (Cheonggukjang) Ameliorates Senile Osteoporosis in the Senescence-Accelerated Mouse Prone 6 Model. J. Med. Food 2019, 22, 1047–1057. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Budryn, G.; Grzelczyk, J.; Pérez-Sánchez, H.; Żyżelewicz, D. Evaluation of Isoflavones as Bone Resorption Inhibitors upon Interactions with Receptor Activator of Nuclear Factor-κB Ligand (RANKL). Molecules 2020, 25, 206. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-F.; Wong, M.-S. Genistein modulates the effects of parathyroid hormone in human osteoblastic SaOS-2 cells. Br. J. Nutr. 2006, 95, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.-K.; Choi, I.-W.; Hong, G.-E.; Pyun, C.-W.; Park, K.-K.; Seo, S.-K.; Lee, C.-H. Effects of Isoflavone Aglycone-rich Fermented Soybean Paste Extracts on Osteoblastic Differentiation of MG-63 Cells. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 803–809. [Google Scholar] [CrossRef]
- Ho, M.-X.; Poon, C.C.-W.; Wong, K.-C.; Qiu, Z.-C.; Wong, M.-S. Icariin, but Not Genistein, Exerts Osteogenic and Anti-apoptotic Effects in Osteoblastic Cells by Selective Activation of Non-genomic ERα Signaling. Front. Pharmacol. 2018, 9, 474. [Google Scholar] [CrossRef] [Green Version]
- Thangavel, P.; Puga-Olguín, A.; Rodríguez-Landa, J.F.; Zepeda, R.C. Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases. Molecules 2019, 24, 3892. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zheng, H.; Qi, S. Genistein and Silicon Synergistically Protects Against Ovariectomy-Induced Bone Loss Through Upregulating OPG/RANKL Ratio. Biol. Trace Element Res. 2018, 188, 441–450. [Google Scholar] [CrossRef]
- Qi, S. Synergistic Effects of Genistein and Zinc on Bone Metabolism and the Femoral Metaphyseal Histomorphology in the Ovariectomized Rats. Biol. Trace Elem. Res. 2017, 183, 288–295. [Google Scholar] [CrossRef]
- Hu, B.; Yu, B.; Tang, D.; Li, S.; Wu, Y. Daidzein promotes osteoblast proliferation and differentiation in OCT1 cells through stimulating the activation of BMP-2/Smads pathway. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Jin, X.; Sun, J.; Yu, B.; Wang, Y.; Sun, W.J.; Yang, J.; Huang, S.H.; Xie, W.L. Daidzein stimulates osteogenesis facilitating proliferation, differentiation, and antiapoptosis in human osteoblast-like MG-63 cells via estrogen receptor–dependent MEK/ERK and PI3K/Akt activation. Nutr. Res. 2017, 42, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, S.; Wang, S.; Gao, P.; Dai, L. A comprehensive review on Pueraria: Insights on its chemistry and medicinal value. Biomed. Pharmacother. 2020, 131, 110734. [Google Scholar] [CrossRef]
- Li, B.; Liu, M.; Wang, Y.; Gong, S.; Yao, W.; Li, W.; Gao, H.; Wei, M. Puerarin improves the bone micro-environment to inhibit OVX-induced osteoporosis via modulating SCFAs released by the gut microbiota and repairing intestinal mucosal integrity. Biomed. Pharmacother. 2020, 132, 110923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, Y.; Tang, G.; Ma, Y. Puerarin inhibits the osteoclastogenesis by inhibiting RANKL-dependent and –independent autophagic responses. BMC Complement. Altern. Med. 2019, 19, 269. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zeng, X.; Zhang, L.; Zheng, X. Stimulatory Effect of Puerarin on Bone Formation through Activation of PI3K/Akt Pathway in Rat Calvaria Osteoblasts. Planta Medica 2007, 73, 341–347. [Google Scholar] [CrossRef]
- Shan, Z.; Cheng, N.; Huang, R.; Zhao, B.; Zhou, Y. Puerarin promotes the proliferation and differentiation of MC3T3-E1 cells via microRNA-106b by targeting receptor activator of nuclear factor-κB ligand. Exp. Ther. Med. 2017, 15, 55–60. [Google Scholar] [CrossRef]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef]
- Jia, M.; Nie, Y.; Cao, D.-P.; Xue, Y.-Y.; Wang, J.-S.; Zhao, L.; Rahman, K.; Zhang, Q.-Y.; Qin, L.-P. Potential Antiosteoporotic Agents from Plants: A Comprehensive Review. Evid.-Based Complement. Altern. Med. 2012, 2012, 364604. [Google Scholar] [CrossRef]
- Zhao, B.-J.; Wang, J.; Song, J.; Gu, J.-F.; Yuan, J.-R.; Zhang, L.; Jiang, J.; Feng, L.; Jia, X.-B. Beneficial Effects of a Flavonoid Fraction of Herba Epimedii on Bone Metabolism in Ovariectomized Rats. Planta Med. 2016, 82, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhou, S.; Qu, R.; Yang, Y.; Gong, X.; Hong, Y.; Jin, A.; Huang, X.; Dai, Q.; Jiang, L. Icariin prevents oestrogen deficiency–induced alveolar bone loss through promoting osteogenesis via STAT3. Cell Prolif. 2020, 53, e12743. [Google Scholar] [CrossRef]
- Keiler, A.; Zierau, O.; Kretzschmar, G. Hop Extracts and Hop Substances in Treatment of Menopausal Complaints. Planta Med. 2013, 79, 576–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milligan, S.R.; Kalita, J.C.; Pocock, V.; Van De Kauter, V.; Stevens, J.F.; Deinzer, M.L.; Rong, H.; De Keukeleire, D. The Endocrine Activities of 8-Prenylnaringenin and Related Hop (Humulus lupulus L.) Flavonoids. J. Clin. Endocrinol. Metab. 2000, 85, 4912–4915. [Google Scholar] [CrossRef]
- Ban, Y.-H.; Yon, J.-M.; Cha, Y.; Choi, J.; An, E.S.; Guo, H.; Seo, D.W.; Kim, T.-S.; Lee, S.-P.; Kim, J.-C.; et al. A Hop Extract Lifenol® Improves Postmenopausal Overweight, Osteoporosis, and Hot Flash in Ovariectomized Rats. Evid.-Based Complement. Altern. Med. 2018, 2018, 2929107. [Google Scholar] [CrossRef] [Green Version]
- Keiler, A.M.; Helle, J.; Bader, M.I.; Ehrhardt, T.; Nestler, K.; Kretzschmar, G.; Bernhardt, R.; Vollmer, G.; Nikolić, D.; Bolton, J.L.; et al. A standardized Humulus lupulus (L.) ethanol extract partially prevents ovariectomy-induced bone loss in the rat without induction of adverse effects in the uterus. Phytomedicine 2017, 34, 50–58. [Google Scholar] [CrossRef]
- Li, J.; Zeng, L.; Xie, J.; Yue, Z.; Deng, H.; Ma, X.; Zheng, C.; Wu, X.; Luo, J.; Liu, M. Inhibition of Osteoclastogenesis and Bone Resorption in vitro and in vivo by a prenylflavonoid xanthohumol from hops. Sci. Rep. 2015, 5, 17605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.M.; Han, E.H.; Jin, Y.H.; Choi, Y.H.; Lee, K.Y.; Jeong, H.G. Xanthohumol from the hop plant stimulates osteoblast differentiation by RUNX2 activation. Biochem. Biophys. Res. Commun. 2011, 409, 82–89. [Google Scholar] [CrossRef]
- Luo, D.; Kang, L.; Ma, Y.; Chen, H.; Kuang, H.; Huang, Q.; He, M.; Peng, W. Effects and mechanisms of 8-prenylnaringenin on osteoblast MC 3T3-E1 and osteoclast-like cells RAW 264.7. Food Sci. Nutr. 2014, 2, 341–350. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Johnson, S.A.; Pourafshar, S.; Navaei, N.; George, K.S.; Hooshmand, S.; Chai, S.C.; Akhavan, N.S. Bone-Protective Effects of Dried Plum in Postmenopausal Women: Efficacy and Possible Mechanisms. Nutrients 2017, 9, 496. [Google Scholar] [CrossRef] [Green Version]
- Hooshmand, S.; Chai, S.C.; Saadat, R.L.; Payton, M.E.; Brummel-Smith, K.; Arjmandi, B.H. Comparative effects of dried plum and dried apple on bone in postmenopausal women. Br. J. Nutr. 2011, 106, 923–930. [Google Scholar] [CrossRef]
- Simonavice, E.; Liu, P.-Y.; Ilich, J.Z.; Kim, J.-S.; Arjmandi, B.; Panton, L.B. The effects of a 6-month resistance training and dried plum consumption intervention on strength, body composition, blood markers of bone turnover, and inflammation in breast cancer survivors. Appl. Physiol. Nutr. Metab. 2014, 39, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Rajput, R.; Wairkar, S.; Gaud, R. Nutraceuticals for better management of osteoporosis: An overview. J. Funct. Foods 2018, 47, 480–490. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Johnson, C.D.; Campbell, S.C.; Hooshmand, S.; Chai, S.C.; Akhter, M.P. Combining Fructooligosaccharide and Dried Plum Has the Greatest Effect on Restoring Bone Mineral Density Among Select Functional Foods and Bioactive Compounds. J. Med. Food 2010, 13, 312–319. [Google Scholar] [CrossRef]
- Bu, S.Y.; Lucas, E.A.; Franklin, M.; Marlow, D.; Brackett, D.J.; Boldrin, E.A.; Devareddy, L.; Arjmandi, B.H.; Smith, B.J. Comparison of dried plum supplementation and intermittent PTH in restoring bone in osteopenic orchidectomized rats. Osteoporos. Int. 2007, 18, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Deyhim, F.; Stoecker, B.J.; Brusewitz, G.H.; Devareddy, L.; Arjmandi, B.H. Dried plum reverses bone loss in an osteopenic rat model of osteoporosis. Menopause 2005, 12, 755–762. [Google Scholar] [CrossRef]
- Rendina, E.; Hembree, K.D.; Davis, M.R.; Marlow, D.; Clarke, S.L.; Halloran, B.P.; Lucas, E.A.; Smith, B.J. Dried Plum’s Unique Capacity to Reverse Bone Loss and Alter Bone Metabolism in Postmenopausal Osteoporosis Model. PLoS ONE 2013, 8, e60569. [Google Scholar] [CrossRef]
- Graef, J.L.; Ouyang, P.; Wang, Y.; Rendina-Ruedy, E.; Lerner, M.R.; Marlow, D.; Lucas, E.A.; Smith, B.J. Dried plum polyphenolic extract combined with vitamin K and potassium restores trabecular and cortical bone in osteopenic model of postmenopausal bone loss. J. Funct. Foods 2018, 42, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.Y.; Lerner, M.; Stoecker, B.J.; Boldrin, E.; Brackett, D.J.; Lucas, E.A.; Smith, B.J. Dried Plum Polyphenols Inhibit Osteoclastogenesis by Downregulating NFATc1 and Inflammatory Mediators. Calcif. Tissue Int. 2008, 82, 475–488. [Google Scholar] [CrossRef]
- Bu, S.Y.; Hunt, T.S.; Smith, B.J. Dried plum polyphenols attenuate the detrimental effects of TNF-α on osteoblast function coincident with up-regulation of Runx2, Osterix and IGF-I. J. Nutr. Biochem. 2009, 20, 35–44. [Google Scholar] [CrossRef]
- Pereira, C.B.; Gomes, P.S.; Costa-Rodrigues, J.; Palmas, R.A.; Vieira, L.; Ferraz, M.P.; Lopes, M.A.; Fernandes, M.H. Equisetum arvense hydromethanolic extracts in bone tissue regeneration: In vitro osteoblastic modulation and antibacterial activity. Cell Prolif. 2012, 45, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rodrigues, J.; Carmo, S.C.; Silva, J.C.; Fernandes, M.H. Inhibition of human in vitro osteoclastogenesis by Equisetum arvense. Cell Prolif. 2012, 45, 566–576. [Google Scholar] [CrossRef]
- Kotwal, S.D.; Badole, S.R. Anabolic therapy with Equisetum arvense along with bone mineralising nutrients in ovariectomized rat model of osteoporosis. Indian J. Pharmacol. 2016, 48, 312–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbabzadegan, N.; Moghadamnia, A.A.; Kazemi, S.; Nozari, F.; Moudi, E.; Haghanifar, S. Effect of Equisetum arvense extract on bone mineral density in Wistar rats via digital radiography. Casp. J. Intern. Med 2019, 10, 176–182. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to Equisetum arvense L. and invigoration of the body (ID 2437), maintenance of skin (ID 2438), maintenance of hair (ID 2438), maintenance of bone (ID 2439), and maintenance or achievement of a normal body weight (ID 2783) pursuant to Article 13 of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1289. [Google Scholar]
- Zepelin, H.-H.H.-V. 60 years of Cimicifuga racemosa medicinal products. Wien. Med. Wochenschr. 2017, 167, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Dragos, D.; Gilca, M.; Gaman, L.; Stoian, I.; Lupescu, O. Osteoprotective medicinal plants-part 1 (A human clinical evidence-based review). Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 102–119. [Google Scholar] [CrossRef] [Green Version]
- Carlisle, A.P.; Jessup, J.V.; Simpson, S.; Yoon, S. Effect of black cohosh on biochemical markers of bone remodeling in postmenopausal women. J. Midwifery Women’s Health 2009, 54, 424. [Google Scholar] [CrossRef]
- Ahn, B.-S.; Yang, M.; Jang, H.; Lee, H.J.; Moon, C.; Kim, J.-C.; Jung, U.; Jo, S.K.; Jang, J.-S.; Kim, S.-H. Evaluation of the Antiosteoporotic Potential of Cimicifuga heracleifolia in Female Mice. Phytother. Res. 2011, 26, 663–668. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; He, C.; Yu, Z.; Du, Y.; Kadota, S.; Seto, H. Triterpenoids from Cimicifugae rhizoma, a novel class of inhibitors on bone resorption and ovariectomy-induced bone loss. Maturitas 2007, 58, 59–69. [Google Scholar] [CrossRef]
- Choi, E.M. Deoxyactein stimulates osteoblast function and inhibits bone-resorbing mediators in MC3T3-E1 cells. J. Appl. Toxicol. 2011, 33, 190–195. [Google Scholar] [CrossRef]
- Lee, Y.S.; Choi, E.M. Actein Isolated from Black Cohosh Promotes the Function of Osteoblastic MC3T3-E1 Cells. J. Med. Food 2014, 17, 414–423. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Xue, L.; Severino, R.P.; Gao, S.; Niu, J.; Qin, L.-P.; Zhang, D.; Brömme, D. Salvia miltiorrhiza: An ancient Chinese herbal medicine as a source for anti-osteoporotic drugs. J. Ethnopharmacol. 2014, 155, 1401–1416. [Google Scholar] [CrossRef]
- Dragos, D.; Gilca, M.; Gaman, L.; Vlad, A.; Iosif, L.; Stoian, I.; Lupescu, O. Phytomedicine in Joint Disorders. Nutrients 2017, 9, 70. [Google Scholar] [CrossRef]
- Kim, M.-H.; Jung, K.; Nam, K.-H.; Jang, H.-J.; Lee, S.W.; Kim, Y.; Park, C.S.; Lee, T.-H.; Park, J.H.; Choi, J.H.; et al. Salvia plebeia R.Br. inhibits signal transduction of IL-6 and prevents ovariectomy-induced bone loss by suppressing osteoclastogenesis. Arch. Pharmacal Res. 2016, 39, 1671–1681. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, R.; Wang, L.; Liu, C.; Ma, R.; Qi, B.; Chen, B.; Li, L.; Guo, Y.; Shi, S.; et al. Radix Salviae miltiorrhizae improves bone microstructure and strength through Wnt/β-catenin and osteoprotegerin/receptor activator for nuclear factor-κB ligand/cathepsin K signaling in ovariectomized rats. Phytother. Res. 2018, 32, 2487–2500. [Google Scholar] [CrossRef]
- Gupta, T.; Das, N.; Imran, S. The Prevention and Therapy of Osteoporosis: A Review on Emerging Trends from Hormonal Therapy to Synthetic Drugs to Plant-Based Bioactives. J. Diet. Suppl. 2018, 16, 699–713. [Google Scholar] [CrossRef]
- Gan, D.; Xu, X.; Chen, D.; Feng, P.; Xu, Z. Network Pharmacology-Based Pharmacological Mechanism of the Chinese Medicine Rhizoma drynariae Against Osteoporosis. Med. Sci. Monit. 2019, 25, 5700–5716. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-A.; Hwang, Y.-H.; Kim, T.; Lee, A.; Ha, H. Anti-Osteoporotic and Anti-Adipogenic Effects of the Water Extract of Drynaria roosii Nakaike in Ovariectomized Mice Fed a High-Fat Diet. Molecules 2019, 24, 3051. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wei, B.; Peng, Z.; Chen, X.; Fu, Q.; Wang, C.; Zhen, J.; Sun, J. A polysaccharide from the dried rhizome of Drynaria fortunei (Kunze) J. Sm. prevents ovariectomized (OVX)-induced osteoporosis in rats. J. Cell. Mol. Med. 2020, 24, 3692–3700. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Wang, G.; Sun, Y.; Deng, Z.; Chen, L.; Chen, K.; Tickner, J.; Kenny, J.; Song, D.; et al. Loureirin B suppresses RANKL-induced osteoclastogenesis and ovariectomized osteoporosis via attenuating NFATc1 and ROS activities. Theranostics 2019, 9, 4648–4662. [Google Scholar] [CrossRef]
- Zou, B.; Zheng, J.; Deng, W.; Tan, Y.; Jie, L.; Qu, Y.; Yang, Q.; Ke, M.; Ding, Z.; Chen, Y.; et al. Kirenol inhibits RANKL-induced osteoclastogenesis and prevents ovariectomized-induced osteoporosis via suppressing the Ca2+-NFATc1 and Cav-1 signaling pathways. Phytomedicine 2021, 80, 153377. [Google Scholar] [CrossRef]
- Nowak, B.; Matuszewska, A.; Nikodem, A.; Filipiak, J.; Landwójtowicz, M.; Sadanowicz, E.; Jędrzejuk, D.; Rzeszutko, M.; Zduniak, K.; Piasecki, T.; et al. Oral administration of kaempferol inhibits bone loss in rat model of ovariectomy-induced osteopenia. Pharmacol. Rep. 2017, 69, 1113–1119. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. The Osteoprotective Effects of Kaempferol: The Evidence From In Vivo And In Vitro Studies. Drug Des. Dev. Ther. 2019, 13, 3497–3514. [Google Scholar] [CrossRef] [Green Version]
- Nowak, B.; Matuszewska, A.; Szandruk, M.; Matkowski, A.; Woźniak, D.; Zduniak, K.; Rzeszutko, M.; Landwójtowicz, M.; Jędrzejuk, D.; Piasecki, T.; et al. Effect of long-term administration of mangiferin from Belamcanda chinensis on bone metabolism in ovariectomized rats. J. Funct. Foods 2018, 46, 12–18. [Google Scholar] [CrossRef]
- Ding, L.-Z.; Teng, X.; Zhang, Z.-B.; Zheng, C.-J.; Chen, S.-H. Mangiferin inhibits apoptosis and oxidative stress via BMP2/Smad-1 signaling in dexamethasone-induced MC3T3-E1 cells. Int. J. Mol. Med. 2018, 41, 2517–2526. [Google Scholar] [CrossRef] [Green Version]
- Sekiguchi, Y.; Mano, H.; Nakatani, S.; Shimizu, J.; Kataoka, A.; Ogura, K.; Kimira, Y.; Ebata, M.; Wada, M. Mangiferin positively regulates osteoblast differentiation and suppresses osteoclast differentiation. Mol. Med. Rep. 2017, 16, 1328–1332. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence. Int. J. Mol. Sci. 2020, 21, 6448. [Google Scholar] [CrossRef]




| Herbal Compounds | Subgroup | Chemical Structure | Proposed Mechanism of Action |
|---|---|---|---|
| Daidzein | isoflavones |  | ER mediated signalling pathway, activation of intracellular pathways: AKT, phospholipase C (PLC), mitogen-activated protein kinase (MAPK) [14] |
| Genistein | isoflavones |  | ER-mediated signalling pathway, activation of intracellular pathways: AKT, PLC, MAPK [14] |
| Ipriflavone | isoflavones |  | Modulation of key signalling pathways to regulate bone resorption (e.g., ↓urinary DPD, NTX) and bone formation (e.g., ↑BALP and osteocalcin [15] |
| Biochanin A | O-methylated isoflavones |  | ER mediated signalling pathway, activation of intracellular pathways: AKT, PLC, MAPK [14] |
| Formononetin | O-methylated isoflavones |  | ER mediated signalling pathway, activation of intracellular pathways: AKT, PLC, MAPK [14] |
| Glycitein | O-methylated isoflavones |  | ER mediated signalling pathway, activation of intracellular pathways: AKT, PLC, MAPK [14] |
| Icariin | prenylated flavonol glycoside |  | Stimulation of bone formation by promotion of osteoblasts differentiation and enhancement of their activity [16]; activation of BMP-2/Smad4, Wnt and IGF-1 signal transduction pathways [5,17], induction of ERK, JNK and p38 kinase activation [18]; decreasing of RANKL-induced osteoclastogenesis via inhibition of NFκB and MAPK expression [19] |
| 8-prenylnaringenin | prenylflavonoids |  | Promotion of osteoblast differentiation and induction of osteoclast apoptosis [20] |
| Epimedin B | prenylflavonoids |  | Inhibition of bone resorption, bone formation promotion and urinary calcium excretion blocking [21] |
| Epimedin C | prenylflavonoids |  | Inhibition of bone resorption, bone formation promotion and urinary calcium excretion blocking [21] |
| Tanshinones (dihydrotanshinone, tanshinone I, or tanshinone IIA) | diterpenes |  Tanshinon 1 Tanshinon 1 | Inhibition of the TRAP5b-expressing osteoclasts formation by suppressing RANKL-induced expression of c-fos and NFATc1 [22,23] |
| Salvianolic acid A | phenolic acids |  | osteoblast differentiation modulation and osteoblast activity upregulation [24,25] |
| Salvianolic acid B | phenolic acids |  | osteoblast differentiation modulation and osteoblast activity upregulation [24,25] |
| Eudebeiolide B | eudesmane-type sesquiterpenoid |  | Osteoclastogenesis inhibition and ovariectomy-induced bone loss prevention by regulating RANKL-Induced NF-κB, c-Fos and Calcium Signaling [26] |
| Botanicals | Population and Design | Intervention | Outcome | Authors and References |
|---|---|---|---|---|
| Soy isoflavones | single open-group prospective clinical intervention; 42 postmenopausal women, | three daily servings for 12 consecutive weeks of whole soy foods containing approximately 60 mg/day of isoflavones | ↓ NTX, ↑ osteocalcin | Scheiber 2001 [27] |
| Soy isoflavones | RCT with 3 groups: soy rich diet, HRT, control; 187 healthy asymptomatic postmenopausal women aged 39–60, | approximately 47 mg/day of isoflavones in diet group; duration: 6 moths | ↑ bone osteoblastic activity but not as effective as HRT in reducing the postmenopausal turnover, ↑ osteocalcin | Chiechi 2002 [28] |
| Soy isoflavones | RCT with 3 groups: placebo, mid-dose, and high-dose, in pill form; 203 postmenopausal Chinese women aged 48 to 62, | placebo (daily dose of 0 mg isoflavones + 500 mg calcium, n = 67) mid-dose (40 mg isoflavones + 500 mg calcium, n = 68) and high-dose (80 mg isoflavones + 500 mg calcium, n = 68); duration: 12months | favourable effect on rates of change in BMC at the total hip and trochanter among later postmenopausal women (>4 y), in women with lower body weight (≤median, 55.5 kg), or among women with lower level of calcium intake (≤median, 1095 mg/day) | Chen 2004 [29] |
| Soy isoflavones | RCT with 3 groups: placebo, mid-dose, and high-dose; 90 Chinese postmenopausal women aged 45–60 | placebo (daily dose of 0 mg isoflavones) mid-dose (84 mg) and high dose (126 mg), 30 subjects/group; duration: 6months | Retardation of lumbar and femoral bone loss at the lumbar spine (L1–L4) and bone resorption | Ye 2006 [30] |
| Soy isoflavones | double-blind RCT with 2 groups: placebo, isoflavone conjugates in capsule form, 68 postmenopausal Japanese women | Isoflavone group (75 mg of isoflavone conjugates/day), 34 subjects/group; duration: 12 months | ↑ serum equol in the equol producers but not in the nonproducers, preventive effects of isoflavones on hip BMD | Wu 2007 [31] |
| Soy isoflavones | double-blind RCT with 3 groups: placebo, mid-dose, and high-dose in tablet form; 255 postmenopausal women aged 46–63 | placebo (daily dose of 0 mg isoflavones) mid-dose (80 mg) and high dose (120 mg); duration: 3 years | mild beneficial femoral BMD—and SSI | Shedd-Wise 2011 [32] |
| Soy isoflavones | double-blind RCT with 2 groups: placebo, isoflavones in tablet form; 87 Korean postmenopausal women aged 45–60 | Isoflavone group = 70 mg in 2 tablet per day (8.0 mg glycitin, 20 mg daidzein, and 12.4 mg genistin); duration: 12 weeks | ↑ serum BALP and osteocalcin | Lee 2017 [33] |
| Soy isoflavones | RCT with 3 groups; placebo, HRT, phytoestrogens; 325 postmenopausal women | HRT group (1 mg oestradiol and 0.5 mg norethisterone acetate p.o. daily, phytoestrogens group (40% standardized extract with 20 mg soy isoflavones (genistein and daidzein), two capsules = 40 mg p.o. daily; duration: 12 months | no significant differences between the effectiveness of the HRT and phytoestrogen in terms of effects on BMD and bone resorption | Tit 2018 [34] |
| Soy isoflavones | double-blind RCT with 3 groups: placebo, soy protein, soy protein + isoflavone in snack bar; 200 women within 2 years of the onset of their menopause | placebo (isoflavone of less than 300 parts per billion) PI (15 g soy protein with 66 mg of isoflavones), SP (15 g soy protein alone, isoflavone free) daily, 100 women/group; duration: 6 months | ↓CTX with SPI supplementation compared to SP, ↓ P1NP with SPI supplementation | Sathyapalan 2017 [35] |
| Soy isoflavones | double-blind RCT with 2 groups: placebo, isoflavones in form of tablet | placebo (0 mg of isoflavones), isoflavones extracted from soy protein (200 mg daily = 4tablets) 248 multi-ethnic menopausal women aged 45 to 60; duration: 2 years | not superior to placebo in preventing bone loss or in reducing bone turnover or menopausal symptoms in women in the first 5 years of menopause | Levis 2011 [36] |
| Soy isoflavones | double-blind RCT with 2 groups: placebo, phytoestrogens; 202 postmenopausal women aged 60–75 | placebo (milk protein), phytoestrogens (25.6 g soy protein containing 52 mg genistein, 41 mg daidzein and 6 mg glycetein (aglycone weights; duration: 12 months | no significant differences for BALP, calcium, and phosphorus measurements. | Kreijkamp-Kaspers 2004 [37] |
| Soy isoflavones | double-blind, multicentre RCT with 2 groups: isoflavone-enriched biscuits and bars and control biscuits and bars; 237 early postmenopausal women aged 53 ± 3y | placebo group (biscuits and cereal bar), isoflavone- enriched foods (soy isoflavone concentrate containing 40–50% of isoflavones) providing a mean daily intake of 110 mg isoflavone aglycones/day; duration: 12 months | isoflavone-enriched products did not alter lumbar and total body BMD or markers of bone formation and bone resorption | Brink 2008 [38] |
| Genistein | double-blind RCT with 2 groups: placebo, genistein; 389 postmenopausal women | placebo group (calcium and vitamin D, n = 191), genistein aglycone group (54 mg/day + calcium and vitamin D, n = 198) duration: 36 months | ↑lumbar and femoral BMD, ↓bone resorption markers (DPD, CTX, RANKL), ↑ bone formation markers (BALP, IGF-1 and OPG) | Marini 2007 [39]; Marini 2008 [40] |
| Genistein | double-blind RCT with 2 groups: placebo, genistein; 138 postmenopausal women (age 49–67 years) | placebo (0mg of isoflavones, n = 67), genistein (54 mg/day, n = 71), duration: 24 months | ↑ femoral and lumbar BMD, improvement of the quantitative ultrasound parameters (stiffness index, amplitude-dependent speed of sound, and bone transmission time) | Atteritano 2009 [41] |
| Genistein | double-blind RCT with 2 groups: placebo, geniVida™ bone blend group; 70 postmenopausal women | placebo (calcium only, n = 28), genistein group = 30 mg/daygenistein + vitamin D3 (800 IU/days) + vitamin K1 (150 μg/days) + polyunsaturated fatty acids (1 g polyunsaturated fatty acids as ethyl ester: eicosapentaenoic acid/docosahexaenoic acid ratio = ~2/1, n = 30); duration: 6 months | ↑ BMD, ↑ BALP and NTX | Lappe 2013 [42] |
| Genistein | double-blind RCT with 2 groups: placebo, genistein, 121 postmenopausal women | placebo (1000 mg of calcium and 800 IU vitamin D3; n = 59), genistein aglycone group (54 mg/day + calcium, vitamin D3; n = 62, duration: 24 months | ↑ femoral and lumbar BMD, ↑ BALP | Arcoraci 2017 [43] |
| Red clover isoflavones (genistein, daidzein, formononetin, biochanin A) | double-blind RCT with 4 groups: placebo, red clover isoflavone preparation (Rimostil) in 3 doses, 46 postmenopausal women | placebo, Rimostil (phytoestrogens)—28.5 mg, 57 mg, or 85.5mg/day, duration: 6 months, | ↑ BMD after 57 mg and 85.5 mg/day | Clifton-Bligh 2001 [44] |
| Red clover isoflavones | double-blind RCT with 2 groups: placebo, isoflavone supplement Promensil®; 205 pre-, peri-, and postmenopausal women aged 49–65 | placebo, isoflavone supplement (providing 26 mg biochanin A, 16 mg formononetin, 1 mg genistein, 0.5 mg daidzein/daily); duration: 12 months | ↑ bone formation markers (BALP, P1NP), ↓ lumbar spine BMC and BMD | Akinson 2004 [45] |
| Red clover isoflavones | double-blind, parallel RCT with 2 groups: placebo, red clover extract; 78 postmenopausal osteopenic women supplemented with calcium 1200 mg/day, magnesium 550 mg/day, calcitriol 25 µg/day | placebo, red clover extract (60 mg isoflavone aglycones/day + probiotics); duration: 12 months | ↓ lumbar and femoral BMD loss, ↓ CTX | Lambert 2017 [46] |
| Red clover isoflavones | double-blind RCT with 2 groups: placebo, red clover extract; 60 menopausal women | placebo, red clover extract (daily dose of 150 mL containing 37.1 mg isoflavones = 33.8 mg as aglycones); duration: 12 weeks | ↑ spinal BMD | Thorup 2015 [47] |
| Red clover isoflavones | double-blind RCT with 2 groups: placebo, standardized red clover isoflavone dietary supplement (Promensil®); 401 healthy women aged 35–70 years | Placebo, red clover isoflavones (40 mg/day); duration: 36 months | safe and well tolerated but no effect on BMD | Powles 2008 [48] |
| Red clover isoflavones | double-blind RCT with 3 groups: placebo and 2 dietary supplements derived from red clover, 252 menopausal women ages 45–60 years | placebo, Promensil® (82 mg total isoflavones), Rimostil® (57.2 mg total isoflavones), duration: 12 weeks | no effect on bone turnover markers. | Knudson Schult 2004 [49] |
| Kudzu root (Pueraria candollei var. mirifica) | double-blind RCT with 4 groups: placebo, 3 dose of Pueraria; 71 postmenopausal women aged 45 to 60 years | placebo (n = 20), Pueraria mirifica in capsules (20, 30, or 50 mg once daily, n = 51); duration: 24 weeks | ↓ BALP | Manonai 2008 [50] |
| Kudzu root (Pueraria candollei var. mirifica) | double-blind RCT with 2 groups 19 postmenopausal women | placebo tablet, tablet containing 25 mg dried PM root powder, 4 tablets/day; duration: 2 months | ↓ ALP | Okamura 2008 [51] |
| Epimedium | double-blind RCT with 2 groups: placebo, Epimedium-derived phytoestrogen flavonoids (EPF), 100 healthy late postmenopausal women | placebo (n = 50), EPF group (n = 50; a daily dose of 60 mg Icariin, 15 mg daidzein, and 3 mg genistein), +300 mg calcium daily for both group; duration: 24 months | ↑ lumbar and femoral BMD, ↓ DPD, | Zang 2007 [52] |
| Dried plums | RCT with 2 groups: placebo (dried apples), dried plums; 58 postmenopausal women | placebo (dried apples 75 g daily), dried plums (100 g daily); duration: 3 months | ↑IGF-1, ↑ ALP, ↑ BALP | Ajamandi 2002 [53] |
| Dried plums | RCT with 2 groups: placebo, dried plums, 160 postmenopausal women with osteopenia | placebo (dried apples 75 g daily), dried plums (100 g daily) + 500 mg Calcium, 400 IU (10 μg) vitamin D daily for both group; duration: 12 months | ↑ ulnar and lumbar BMD, ↓ BALP | Hooshmand 2011 [54] |
| Dried plums | RCT with 3 groups: placebo, 2 dose of dried plums, 48 older postmenopausal women | control (0 g/day dried plum), dried plum (50 or 100 g/day dried plum), duration: 6 months | ↑ BMD, ↓ TRAP-5b, ↑ BALP/TRAP-5b ratio | Hooshmand 2016 [55] |
| Dried plums | RCT with 3 groups: placebo, 2 dose of dried plums; 35 men between the ages of 55 and 80 with moderate bone loss | control group (0g prunes), 100 g prunes daily, 50 g prunes daily, + multivitamin containing 450 mg calcium and 800 IU vitamin D for all group, duration: 3 months | ↓ osteocalcin, ↑ OPG/RANKL ratio | Ajmandi 2020 [56] |
| Horsetail (Equisetum arvense) | Double blind RCT with 4 groups: control, placebo + horsetail extract, horsetail extract, calcium, 122 women in menopause for at least two years | no treatment/control group (n = 29), placebo for 40 days and titrated horsetail extract for a further 40 days (n = 31), titrated dry horsetail extract for 80 days (n = 30); Calcium (Osteosil®) for 80 days (n = 32), After the 80-day initial study period, patients treated with titrated horsetail extract and with calcium continued treatment for one year | ↑ in the average densitometric values for the vertebra | Corletto 1999 [57] |
| Black cohosh (Cimcifuga racemosa) | double-blind RCT with 3 groups: placebo, black cohosh, oestrogens; 62 postmenopausal women | placebo, black cohosh (40 mg of herbal drug/day), conjugated oestrogens (0.6 mg/day); duration: 12 weeks. | ↑ osteoblast activity, weak estrogen-like activity, no significant effects on coagulation markers and liver enzymes | Wuttke 2006 [58] |
| Black cohosh (Cimcifuga racemosa) | prospective clinical trial with 2 groups: untreated control, isopropanolic extract of Cimicifuga racemosa, 82 postmenopausal women | control group (n = 37), isopropanolic extract of Cimicifuga racemosa (Remifemin®, 40 mg/day, n = 45), duration: 3 months | ↓NTX (marker of bone resorption), ↑ ALP (marker of bone formation) | Garcia-Pérez 2009 [59] |
| Black cohosh (Cimcifuga racemosa) | RCT with 3 groups: control (CG), exercise group (EG), exercise and Cimicifuga racemosa (CR) supplementation group (EGCR), 128 early postmenopausal women | CG (wellness control, n = 42), EG (n = 43), EGCR (40 mg/day of CR BNO 1055; n = 43), Calcium (1500 mg/d) + vitamin D (500 IE/d) supplementation for all participant duration:12 months | CR (CR BNO 1055) did not enhance positive effects of exercise on BMD at the lumbar spine | Bebenek 2010 [60] |
| Labisia pumila and Eurycoma longifolia | double-blind RCT with 2 groups: placebo, Nu-femme™, 119 healthy women aged 41–55 years experiencing peri-menopausal or menopausal symptoms | placebo (n = 59), herbal formulation (Nu-femme™, n = 60) = 200mg Labisia pumila (SLP+®) + 50mg Eurycoma longifolia (Physta®); duration: 24 weeks | No significant effect on bone formation (BALP) and resorption (NTX) markers | Chinnappan 2020 [61] |
| Aglycones | Glycosides | Acetylglycosides | Malonyl Isoflavone Glycosides |
|---|---|---|---|
| Daidzein | Daidzin | Acetyldaidzin | Malonyldaidzin |
| Genistein | Genistin | Acetylgenistin | Malonylgenistin |
| Glycitein | Glycitin | Acetylglycitin | Malonylglycitin |
| Biochanin A | Sissostrin | Malonylsissostrin | |
| Formononetin | Ononin | Malonylononin | |
| Daidzein | Daidzin | Acetyldaidzin | Malonyldaidzin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słupski, W.; Jawień, P.; Nowak, B. Botanicals in Postmenopausal Osteoporosis. Nutrients 2021, 13, 1609. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13051609
Słupski W, Jawień P, Nowak B. Botanicals in Postmenopausal Osteoporosis. Nutrients. 2021; 13(5):1609. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13051609
Chicago/Turabian StyleSłupski, Wojciech, Paulina Jawień, and Beata Nowak. 2021. "Botanicals in Postmenopausal Osteoporosis" Nutrients 13, no. 5: 1609. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13051609