Dietary Intake of Vitamin E and Fats Associated with Sarcopenia in Community-Dwelling Older Japanese People: A Cross-Sectional Study from the Fifth Survey of the ROAD Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Interviewer-Administered Questionnaire and Anthropometric Measurements
2.3. Walking Speed, Muscle Strength, and Skeletal Muscle Mass
2.4. Definition of Sarcopenia
2.5. Dietary Assessment
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Differences between Intakes of Nutrients with and without Sarcopenia
3.3. The Prevalence of Sarcopenia with the Amount of Vitamin E and Fats Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; González-Gálvez, N.; López-Vivancos, A.; Espeso-García, A.; Martínez-Aranda, L.M.; Gea-García, G.M.; Orquín-Castrillón, F.J.; Carbonell-Baeza, A.; Jiménez-García, J.D.; Velázquez-Díaz, D.; et al. Sarcopenia, Diet, Physical Activity and Obesity in European Middle-Aged and Older Adults: The LifeAge Study. Nutrients 2020, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Bloom, I.; Shand, C.; Cooper, C.; Robinson, S.; Baird, J. Diet Quality and Sarcopenia in Older Adults: A Systematic Review. Nutrients 2018, 10, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Vatic, M.; von Haehling, S.; Ebner, N. Inflammatory biomarkers of frailty. Exp. Gerontol 2020, 133, 110858. [Google Scholar] [CrossRef] [PubMed]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- American College of Sports Medicine Position Stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Tessier, A.J.; Chevalier, S. An Update on Protein, Leucine, Omega-3 Fatty Acids, and Vitamin D in the Prevention and Treatment of Sarcopenia and Functional Decline. Nutrients 2018, 10, 1099. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.; Granic, A.; Sayer, A.A. Nutrition and Muscle Strength, As the Key Component of Sarcopenia: An Overview of Current Evidence. Nutrients 2019, 11, 2942. [Google Scholar] [CrossRef] [Green Version]
- Mareschal, J.; Genton, L.; Collet, T.H.; Graf, C. Nutritional Intervention to Prevent the Functional Decline in Community-Dwelling Older Adults: A Systematic Review. Nutrients 2020, 12, 2820. [Google Scholar] [CrossRef]
- Dreyer, H.C.; Drummond, M.J.; Pennings, B.; Fujita, S.; Glynn, E.L.; Chinkes, D.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E392–E400. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.Y.; Schutzler, S.; Schrader, A.; Spencer, H.; Kortebein, P.; Deutz, N.E.; Wolfe, R.R.; Ferrando, A.A. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E21–E28. [Google Scholar] [CrossRef]
- Tagawa, R.; Watanabe, D.; Ito, K.; Ueda, K.; Nakayama, K.; Sanbongi, C.; Miyachi, M. Dose-response relationship between protein intake and muscle mass increase: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2020, 79, 66–75. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Punzi, L.; Soysal, P.; Incalzi, R.A.; Saller, A.; Maggi, S. Effect of nutritional supplementations on physical performance and muscle strength parameters in older people: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 51, 48–54. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Milano-Teixeira, L.; Rodrigues, B.; Bacurau, R.; Marzetti, E.; Uchida, M. Relative Protein Intake and Physical Function in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 1300. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.; Tokuda, Y. Differences and overlap between sarcopenia and physical frailty in older community-dwelling Japanese. Asia Pac. J. Clin. Nutr. 2019, 28, 157–165. [Google Scholar] [CrossRef]
- Suthuvoravut, U.; Takahashi, K.; Murayama, H.; Tanaka, T.; Akishita, M.; Iijima, K. Association between Traditional Japanese Diet Washoku and Sarcopenia in Community-Dwelling Older Adults: Findings from the Kashiwa Study. J. Nutr. Health Aging 2020, 24, 282–289. [Google Scholar] [CrossRef]
- Yoshimura, N.; Muraki, S.; Oka, H.; Mabuchi, A.; En-Yo, Y.; Yoshida, M.; Saika, A.; Yoshida, H.; Suzuki, T.; Yamamoto, S.; et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: The research on osteoarthritis/osteoporosis against disability study. J. Bone Min. Metab. 2009, 27, 620–628. [Google Scholar] [CrossRef]
- Muraki, S.; Oka, H.; Akune, T.; Mabuchi, A.; En-yo, Y.; Yoshida, M.; Saika, A.; Suzuki, T.; Yoshida, H.; Ishibashi, H.; et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: The ROAD study. Osteoarthr. Cartil. 2009, 17, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, N.; Muraki, S.; Oka, H.; Iidaka, T.; Kodama, R.; Kawaguchi, H.; Nakamura, K.; Tanaka, S.; Akune, T. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int. 2017, 28, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Muraki, S.; Iidaka, T.; Oka, H.; Horii, C.; Kawaguchi, H.; Akune, T.; Nakamura, K.; Tanaka, S. Prevalence and co-existence of locomotive syndrome, sarcopenia, and frailty: The third survey of Research on Osteoarthritis/Osteoporosis Against Disability (ROAD) study. J. Bone Min. Metab. 2019, 37, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Akune, T.; Muraki, S.; Oka, H.; Tanaka, S.; Kawaguchi, H.; Nakamura, K.; Yoshimura, N. Exercise habits during middle age are associated with lower prevalence of sarcopenia: The ROAD study. Osteoporos Int. 2014, 25, 1081–1088. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, M.; Yabushita, N.; Kim, M.; Matsuo, T.; Seino, S.; Songee, J.; Sasai, H.; Tanaka, K. Validity of the bioelectrical impedance method for assessing body composition in non-frail and pre-frail older adults. Int. J. Body Compos. Res. 2012, 10, 55–61. [Google Scholar]
- Sasaki, S.; Yanagibori, R.; Amano, K. Self-administered diet history questionnaire developed for health education: A relative validation of the test-version by comparison with 3-day diet record in women. J. Epidemiol. 1998, 8, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Muraki, S.; Akune, T.; En-yo, Y.; Yoshida, M.; Tanaka, S.; Kawaguchi, H.; Nakamura, K.; Oka, H.; Yoshimura, N. Association of dietary intake with joint space narrowing and osteophytosis at the knee in Japanese men and women: The ROAD study. Mod. Rheumatol. 2014, 24, 236–242. [Google Scholar] [CrossRef]
- Odai, T.; Terauchi, M.; Hirose, A.; Kato, K.; Miyasaka, N. Bone Mineral Density in Premenopausal Women Is Associated with the Dietary Intake of α-Tocopherol: A Cross-Sectional Study. Nutrients 2019, 11, 2474. [Google Scholar] [CrossRef] [Green Version]
- Japan Sports Agency. The Report of Physical and Exercise Capacity. 2018. Available online: https://www.mext.go.jp/prevsports/comp/bmenu/other/icsFiles/afieldfile/2019/10/15/14219212.pdf (accessed on 25 March 2021).
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Chung, E.; Mo, H.; Wang, S.; Zu, Y.; Elfakhani, M.; Rios, S.R.; Chyu, M.C.; Yang, R.S.; Shen, C.L. Potential roles of vitamin E in age-related changes in skeletal muscle health. Nutr. Res. 2018, 49, 23–36. [Google Scholar] [CrossRef]
- Aragno, M.; Mastrocola, R.; Catalano, M.G.; Brignardello, E.; Danni, O.; Boccuzzi, G. Oxidative stress impairs skeletal muscle repair in diabetic rats. Diabetes 2004, 53, 1082–1088. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Xing, Q.; Li, Y.; Han, X.; Sun, L. Dexmedetomidine protects against ischemia-reperfusion injury in rat skeletal muscle. J. Surg. Res. 2014, 186, 240–245. [Google Scholar] [CrossRef]
- Servais, S.; Letexier, D.; Favier, R.; Duchamp, C.; Desplanches, D. Prevention of unloading-induced atrophy by vitamin E supplementation: Links between oxidative stress and soleus muscle proteolysis? Free Radic. Biol. Med. 2007, 42, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Bartali, B.; Frongillo, E.A.; Guralnik, J.M.; Stipanuk, M.H.; Allore, H.G.; Cherubini, A.; Bandinelli, S.; Ferrucci, L.; Gill, T.M. Serum micronutrient concentrations and decline in physical function among older persons. JAMA 2008, 299, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, A.A.; Hayhoe, R.P.G.; Luben, R.N.; Welch, A.A. Positive Associations of Dietary Intake and Plasma Concentrations of Vitamin E with Skeletal Muscle Mass, Heel Bone Ultrasound Attenuation and Fracture Risk in the EPIC-Norfolk Cohort. Antioxidants 2021, 10, 159. [Google Scholar] [CrossRef]
- Welch, A.A.; Jennings, A.; Kelaiditi, E.; Skinner, J.; Steves, C.J. Cross-Sectional Associations Between Dietary Antioxidant Vitamins C, E and Carotenoid Intakes and Sarcopenic Indices in Women Aged 18–79 Years. Calcif. Tissue Int. 2020, 106, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Overview of Dietary Reference Intakes for Japanese 2015. Available online: http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Overview.pdf (accessed on 25 March 2021).
- Li, C.; Kang, B.; Zhang, T.; Gu, H.; Song, P.; Chen, J.; Wang, X.; Xu, B.; Zhao, W.; Zhang, J. Dietary Pattern and Dietary Energy from Fat Associated with Sarcopenia in Community-Dwelling Older Chinese People: A Cross-Sectional Study in Three Regions of China. Nutrients 2020, 12, 3689. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; MacGregor, A.J.; Minihane, A.M.; Skinner, J.; Valdes, A.A.; Spector, T.D.; Cassidy, A. Dietary fat and fatty acid profile are associated with indices of skeletal muscle mass in women aged 18-79 years. J. Nutr. 2014, 144, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Alhussain, M.H.; Alkahtani, S.; Aljuhani, O.; Habib, S.S. Effects of Nutrient Intake on Diagnostic Measures of Sarcopenia among Arab Men: A Cross-Sectional Study. Nutrients 2020, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Locquet, M.; Touvier, M.; Reginster, J.Y.; Bruyère, O. Association between dietary nutrient intake and sarcopenia in the SarcoPhAge study. Aging Clin. Exp. Res. 2019, 31, 815–824. [Google Scholar] [CrossRef]
- Huang, Y.H.; Chiu, W.C.; Hsu, Y.P.; Lo, Y.L.; Wang, Y.H. Effects of Omega-3 Fatty Acids on Muscle Mass, Muscle Strength and Muscle Performance among the Elderly: A Meta-Analysis. Nutrients 2020, 12, 3739. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Montorfano, G.; Klersy, C.; Massimino, L.; Infantino, V.; Iannello, G.; Anna Faliva, M.; Lukaski, H.; Perna, S.; Alalwan, T.A.; et al. Fatty Acid Profile and Antioxidant Status Fingerprint in Sarcopenic Elderly Patients: Role of Diet and Exercise. Nutrients 2019, 11, 2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lim, J.Y.; Choi, S.J. Oleate Prevents Palmitate-Induced Atrophy via Modulation of Mitochondrial ROS Production in Skeletal Myotubes. Oxid. Med. Cell Longev. 2017, 2017, 2739721. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhang, F.; Ji, X.; Yu, H.; Jiang, X.; Qiu, Y.; Yu, J.; Chen, J.; Yang, F.; Bao, Z. Oleate Ameliorates Palmitate-Induced Impairment of Differentiative Capacity in C2C12 Myoblast Cells. Stem. Cells Dev. 2021, 30, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Højfeldt, G.; Nishimura, Y.; Mertz, K.; Schacht, S.R.; Lindberg, J.; Jensen, M.; Hjulmand, M.; Lind, M.V.; Jensen, T.; Jespersen, A.P.; et al. Daily Protein and Energy Intake Are Not Associated with Muscle Mass and Physical Function in Healthy Older Individuals-A Cross-Sectional Study. Nutrients 2020, 12, 2794. [Google Scholar] [CrossRef]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Distribution but not amount of protein intake is associated with frailty: A cross-sectional investigation in the region of Nürnberg. Nutr. J. 2013, 12, 109. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Cumming, R.G.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Ribeiro, R.V.; Simpson, S.J.; Hirani, V. Associations between nutrient intakes and dietary patterns with different sarcopenia definitions in older Australian men: The concord health and ageing in men project. Public Health Nutr. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. The Report of National Health and Nutrition Survey (2018). Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/h30-houkoku_00001.html (accessed on 25 March 2021).

| Characteristic | Overall (n = 1345) | Men (n = 437) | Women (n = 908) | p Value * |
|---|---|---|---|---|
| Age (y) | 71.2 (7.4) | 71.4 (7.9) | 71.1 (7.1) | 0.513 |
| Height (cm) | 156.1 (8.9) | 165.3 (6.3) | 151.7 (6.1) | <0.001 |
| Weight (cm) | 55.6 (10.9) | 63.8 (10.8) | 51.7 (8.5) | <0.001 |
| BMI (kg/m2) | 22.7 (3.4) | 23.3 (3.4) | 22.5 (3.4) | <0.001 |
| Residing in a coastal area (%) | 54.9 | 53.1 | 55.8 | 0.343 |
| Current smoking habit (%) | 7.0 | 16.5 | 2.4 | <0.001 |
| Current alcohol drinking habit (%) | 40.5 | 66.6 | 28.0 | <0.001 |
| Usual walking speed (m/s) | 1.17 (0.26) | 1.16 (0.25) | 1.18 (0.26) | 0.140 |
| Handgrip strength (kg) | 29.1 (9.5) | 39.6 (7.8) | 24.0 (4.8) | <0.001 |
| Appendicular muscle mass index (kg/m2) | 6.6 (1.1) | 7.7 (1.1) | 6.0 (0.6) | <0.001 |
| Prevalence of sarcopenia (%) | 5.7 | 5.0 | 6.1 | 0.450 |
| Category | Nutrient Factor | Sarcopenia (+) (n = 77) | Sarcopenia (−) (n = 1268) | p Value * |
|---|---|---|---|---|
| Calorie | Total energy (kcal/day) | 1808.4 (522.2) | 1877.8 (559.4) | 0.289 |
| Major nutrient | Protein (g/day) | 73.3 (27.4) | 75.9 (28.2) | 0.433 |
| Animal protein (g/day) | 44.8 (21.8) | 46.6 (22.4) | 0.493 | |
| Vegetable protein (g/day) | 28.5 (8.6) | 29.3 (9.2) | 0.462 | |
| Fat (g/day) | 50.6 (19.2) | 55.7 (20.6) | 0.032 | |
| Animal fat (g/day) | 25.4 (12.1) | 27.3 (12.2) | 0.181 | |
| Vegetable fat (g/day) | 25.2 (10.2) | 28.4 (11.1) | 0.013 | |
| Carbohydrate (g/day) | 249.6 (72.0) | 247.5 (79.3) | 0.823 | |
| Mineral | Ash (g/day) | 18.8 (6.4) | 19.6 (6.5) | 0.268 |
| Sodium (mg/day) | 4314.7 (1385.3) | 4483.4 (1506.3) | 0.338 | |
| Potassium (mg/day) | 2557.4 (1039.6) | 2709.8 (1016.6) | 0.202 | |
| Calcium (mg/day) | 604.8 (279.3) | 613.1 (261.3) | 0.788 | |
| Magnesium (mg/day) | 253.7 (94.4) | 268.8 (94.7) | 0.175 | |
| Phosphorus (mg/day) | 1124.6 (434.7) | 1163.6 (436.6) | 0.447 | |
| Iron (mg/day) | 7.8 (3.1) | 8.1 (3.1) | 0.382 | |
| Zinc (mg/day) | 8.1 (2.7) | 8.4 (2.8) | 0.400 | |
| Copper (mg/day) | 1.1 (0.4) | 1.2 (0.4) | 0.717 | |
| Manganese (mg/day) | 3.2 (1.1) | 3.2 (1.1) | 0.825 | |
| Fat-soluble vitamin | α-Carotene (μg/day) | 465.4 (440.0) | 421.1 (328.5) | 0.261 |
| β-Carotene (μg/day) | 3165.3 (2648.5) | 3098.7 (2036.6) | 0.784 | |
| Retinol (μg/day) | 474.5 (502.9) | 444.1 (404.2) | 0.527 | |
| Cryptoxanthin (μg/day) | 448.7 (400.0) | 505.7 (402.8) | 0.228 | |
| β-Carotene equivalents (μg/day) | 3627.0 (2919.5) | 3567 (2246.2) | 0.823 | |
| Retinol equivalents (μg/day) | 780.5 (587.9) | 745.2 (485.7) | 0.542 | |
| Vitamin D (μg/day) | 21.3 (14.6) | 20.8 (14.3) | 0.770 | |
| α-Tocopherol (mg/day) | 7.2 (3.1) | 7.9 (3.1) | 0.049 | |
| β-Tocopherol (mg/day) | 0.3 (0.1) | 0.4 (0.1) | 0.004 | |
| γ-Tocopherol (mg/day) | 11.0 (4.7) | 12.7 (5.1) | 0.003 | |
| δ-Tocopherol (mg/day) | 2.8 (1.1) | 3.1 (1.2) | 0.033 | |
| Vitamin K (μg/day) | 256.1 (165.1) | 269.5 (165.2) | 0.489 | |
| Water-soluble vitamin | Vitamin B1 (mg/day) | 0.8 (0.3) | 0.8 (0.3) | 0.143 |
| Vitamin B2 (mg/day) | 1.3 (0.5) | 1.4 (0.5) | 0.438 | |
| Niacin (mg/day) | 17.6 (7.6) | 19.2 (7.7) | 0.077 | |
| Vitamin B6 (mg/day) | 1.3 (0.5) | 1.4 (0.5) | 0.308 | |
| Vitamin B12 (μg/day) | 12.6 (7.8) | 12.8 (7.8) | 0.844 | |
| Folic acid (μg/day) | 330.3 (154.9) | 348.5 (145.6) | 0.287 | |
| Pantothenic acid (mg/day) | 6.6 (2.4) | 6.8 (2.4) | 0.573 | |
| Vitamin C (mg/day) | 125.6 (65.7) | 135.2 (69.2) | 0.235 | |
| Fatty acid | Saturated fatty acid (g/day) | 13.6 (5.5) | 14.7 (5.8) | 0.105 |
| Monounsaturated fatty acid (g/day) | 17.6 (7.2) | 19.8 (7.6) | 0.016 | |
| Polyunsaturated fatty acid (g/day) | 12.0 (4.5) | 13.4 (5.1) | 0.014 | |
| n-3 fatty acid (g/day) | 2.9 (1.4) | 3.1 (1.5) | 0.333 | |
| n-6 fatty acid (g/day) | 9.0 (3.3) | 10.3 (3.8) | 0.004 | |
| Others | Cholesterol (mg/day) | 420.0 (199.7) | 436.7 (200.6) | 0.479 |
| Soluble dietary fiber (g/day) | 2.9 (1.3) | 3.1 (1.3) | 0.194 | |
| Insoluble dietary fiber (g/day) | 8.5 (3.4) | 8.9 (3.4) | 0.241 | |
| Dietary fiber (g/day) | 11.8 (4.9) | 12.4 (4.9) | 0.319 | |
| Salt equivalent (g/day) | 10.9 (3.5) | 11.3 (3.8) | 0.341 | |
| Sucrose (g/day) | 13.9 (9.2) | 14.2 (9.8) | 0.845 | |
| Alcohol (g/day) | 5.4 (14.9) | 8.7 (18.9) | 0.127 | |
| Daidzein (mg/day) | 12.5 (9.0) | 12.4 (9.3) | 0.987 | |
| Genistein (mg/day) | 21.2 (15.3) | 21.2 (15.8) | 0.986 |
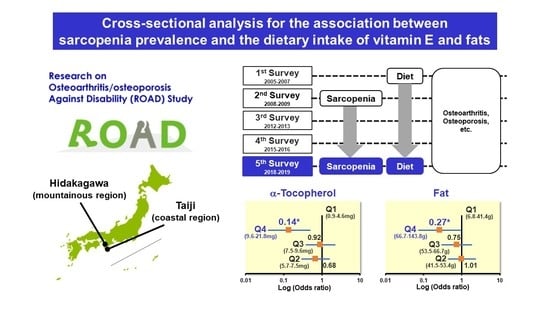
| Nutrient Factor | Characteristic | Daily Intake | Trend p * | |||
|---|---|---|---|---|---|---|
| Q1 (Low) | Q2 | Q3 | Q4 (High) | |||
| Fat | Median (range) of intake (g/day) † | 33.8 (6.8–41.4) | 47.5 (41.5–53.4) | 59.2 (53.5–66.7) | 77.9 (66.7–143.8) | |
| N with or without sarcopenia ‡ | 23/313 | 21/316 | 22/314 | 11/325 | ||
| Adjusted OR (95% CI) § | 1.00 (reference) | 1.01 (0.45–2.27) | 0.75 (0.31–1.82) | 0.27 (0.08–0.96) | 0.037 | |
| Vegetable fat | Median (range) of intake (g/day) † | 16.5 (1.9–20.5) | 24.1 (20.5–27.1) | 30.2 (27.1–34.6) | 40.0 (34.6–75.8) | |
| N with or without sarcopenia ‡ | 27/309 | 20/317 | 17/319 | 13/323 | ||
| Adjusted OR (95% CI) § | 1.00 (reference) | 0.86 (0.40–1.83) | 0.67 (0.28–1.58) | 0.53 (0.18–1.50) | 0.202 | |
| α-Tocopherol | Median (range) intake (mg/day) † | 4.6 (0.9–5.7) | 6.6 (5.7–7.5) | 8.4 (7.5–9.6) | 11.2 (9.6–21.8) | |
| N with or without sarcopenia ‡ | 25/311 | 17/320 | 26/310 | 9/327 | ||
| Adjusted OR (95% CI) § | 1.00 (reference) | 0.68 (0.30–1.55) | 0.92 (0.38–2.19) | 0.14 (0.04–0.49) | 0.008 | |
| β-Tocopherol | Median (range) intake (mg/day) † | 0.21 (0.02–0.27) | 0.31 (0.27–0.35) | 0.39 (0.35–0.44) | 0.51 (0.44–0.95) | |
| N with or without sarcopenia ‡ | 24/312 | 24/312 | 22/315 | 7/329 | ||
| Adjusted OR (95% CI) § | 1.00 (reference) | 0.93 (0.44–1.94) | 0.78 (0.34–1.78) | 0.24 (0.07–0.78) | 0.018 | |
| γ-Tocopherol | Median (range) intake (mg/day) † | 7.2 (0.9–9.2) | 10.6 (9.2–12.0) | 13.7 (12.0–15.4) | 18.2 (15.4–36.1) | |
| N with or without sarcopenia ‡ | 25/311 | 22/314 | 23/314 | 7/329 | ||
| Adjusted OR (95% CI) § | 1.00 (reference) | 1.31 (0.62–2.77) | 1.07 (0.49–2.38) | 0.28 (0.09–0.87) | 0.024 | |
| δ-Tocopherol | Median (range) intake (mg/day) † | 1.7 (0.3–2.2) | 2.6 (2.2–2.9) | 3.3 (2.9–3.8) | 4.4 (3.8–8.5) | |
| N with or without sarcopenia ‡ | 22/314 | 24/313 | 20/316 | 11/325 | ||
| Adjusted OR (95% CI) § | 1.00 (reference) | 1.60 (0.75–3.42) | 1.09 (0.47–2.55) | 0.75 (0.26–2.12) | 0.409 | |
| Monounsaturated fatty acid | Median (range) intake (g/day) † | 11.8 (2.0–14.5) | 16.5 (14.6–18.8) | 20.9 (18.8–24.0) | 28.0 (24.0–53.3) | |
| N with or without sarcopenia ‡ | 25/311 | 22/315 | 19/317 | 11/325 | ||
| Adjusted OR (95% CI) § | 1.00 (reference) | 0.94 (0.43–2.05) | 0.69 (0.29–1.67) | 0.22 (0.07–0.72) | 0.011 | |
| Polyunsaturated fatty acid | Median (range) intake (g/day) † | 8.0 (1.6–9.9) | 11.4 (10.0–12.7) | 14.2 (12.7–16.0) | 18.9 (16.0–34.1) | |
| N with or without sarcopenia ‡ | 23/313 | 22/314 | 22/315 | 10/326 | ||
| Adjusted OR (95% CI) § | 1.00 (reference) | 1.19 (0.54–2.63) | 0.96 (0.41–2.23) | 0.28 (0.09–0.89) | 0.029 | |
| n-6 fatty acid | Median (range) intake (g/day) † | 6.2 (0.9–7.6) | 8.7 (7.6–9.8) | 10.9 (9.8–12.2) | 14.3 (12.2–26.3) | |
| N with or without sarcopenia ‡ | 23/313 | 26/310 | 20/317 | 8/328 | ||
| Adjusted OR (95% CI) § | 1.00 (reference) | 1.65 (0.77–3.51) | 1.01 (0.42–2.39) | 0.39 (0.12–1.26) | 0.064 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otsuka, Y.; Iidaka, T.; Horii, C.; Muraki, S.; Oka, H.; Nakamura, K.; Izumo, T.; Rogi, T.; Shibata, H.; Tanaka, S.; et al. Dietary Intake of Vitamin E and Fats Associated with Sarcopenia in Community-Dwelling Older Japanese People: A Cross-Sectional Study from the Fifth Survey of the ROAD Study. Nutrients 2021, 13, 1730. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13051730
Otsuka Y, Iidaka T, Horii C, Muraki S, Oka H, Nakamura K, Izumo T, Rogi T, Shibata H, Tanaka S, et al. Dietary Intake of Vitamin E and Fats Associated with Sarcopenia in Community-Dwelling Older Japanese People: A Cross-Sectional Study from the Fifth Survey of the ROAD Study. Nutrients. 2021; 13(5):1730. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13051730
Chicago/Turabian StyleOtsuka, Yuta, Toshiko Iidaka, Chiaki Horii, Shigeyuki Muraki, Hiroyuki Oka, Kozo Nakamura, Takayuki Izumo, Tomohiro Rogi, Hiroshi Shibata, Sakae Tanaka, and et al. 2021. "Dietary Intake of Vitamin E and Fats Associated with Sarcopenia in Community-Dwelling Older Japanese People: A Cross-Sectional Study from the Fifth Survey of the ROAD Study" Nutrients 13, no. 5: 1730. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13051730